1. Background
Very much research has been done to characterize natural substances which might control fungal growth and aflatoxin production. In order to inhibit aflatoxin production, some natural products are known. Aflatoxins B1, B2, G1 and G2, are extremely toxic, carcinogenic, mutagenic compounds generally produced by important species of fungi such as Aspergillus flavus, Aspergillus parasiticus and Aspergillus nomius in various food commodities (1, 2). Aflatoxin B1 is considered a group A carcinogen by international agencies (3, 4). Among aflatoxins produced by Aspergillus parasiticus are aflatoxin B & G. Nevertheless, aflatoxin B1 (AFB1) is a very powerful carcinogen to humans and animals, which is very well recognized (5, 6). Although a global issue, aflatoxin food pollution is particularly problematic in the subtropical area and tropical area of the world where humidity and ambient temperature favors the growth of the fungi (7). Satureja rechingeri is a member of the large Lamiaceae family. Its relationship with Satureja Edmund and Satureja khuzestanica has been reviewed and discussed (8).
2. Objectives
In this study, efforts were made to evaluate growth inhibition of fungi of Aspergillus species by the essential oil of Satureja rechingeri.
3. Methods
3.1. Cultures and Fungal Strains
The microorganism was maintained on Sabouraud dextrose agar (Quelab, Canada). Spore suspensions of Aspergillus were prepared and diluted with sterile water yeast extract sucrose (YES) broth, at a concentration of about 107 and 105 spores/mL. Spore population was counted using a haemocytometer (9). Aspergillus parasiticus NRRL2999 and Aspergillus flavus PICC-AF39 were used.
3.2. Plant Materials
S. rechingeri essential oil was purchased from Khoraman Co. (Khorramabad, Iran).
3.3. Disk Diffusion Assay
Antifungal screening by diffusion method was as follows: after sterilizing Sabouraud dextrose agar (Que Lab), then distributed into petri dishes with a diameter of 80 mm, Aspergillus parasiticus NRRL2999 and Aspergillus flavus PICC-AF39, spores (105 and 107 spores/mL) inoculum on the surface. Sterile filter paper 6-mm Whitman No. 1 was used as the disc the Whitman discs were placed on the agar plates containing Aspergillus and then of different concentrations of essential oils 1, 2, 3, 4, 5, 10, and 20 μL of oils was put on the discs, all was done under aseptic conditions. The plates inoculated with spores were incubated for 10 days at temperature of 28 ± 2ºC. Three replicates were used for each treatment. Using Vernier calipers, the diameter of the microbial inhibition zones was measured (10).
3.4. The Minimal Inhibitory Concentration and Minimal Fungicidal Concentration
Varying amounts of concentration of the oils were added to 2 mL of yeast broth tubes containing 100 µL, 105 and 107 spores/mL in order to evenly disperse the oil throughout the broth inside of tubes and the tubes stayed incubated for 72 hours on a shaker incubator. As the minimal inhibitory concentration (MIC) is the highest dilutions that prevent visible growth was regarded, which can be determined by culturing microorganisms from tubes not growth on plates of solid Sabouraud dextrose agar. The highest dilutions at which no growth happened on the plates was determined as the minimal fungicidal concentration (MFC).
3.5. Reaction Kinetics Fungicidal by Satureja rechingeri
Triplicate of S. rechingeri oil at the MFC dilution was added to 2 mL of spore suspension tubes comprising 105 and 107 spores/mL incubated at 28 ± 2ºC in a shaker incubator in times 0, 15, 30, 60, 90, 120, 150, 180 and 210 min. Samples taken were cultured on solid Sabouraud dextrose agar plates at temperature of 28 ± 2ºC for 48 h. No essential oil was added to the control tubes. After the incubation period fungal colonies were counted.
4. Results
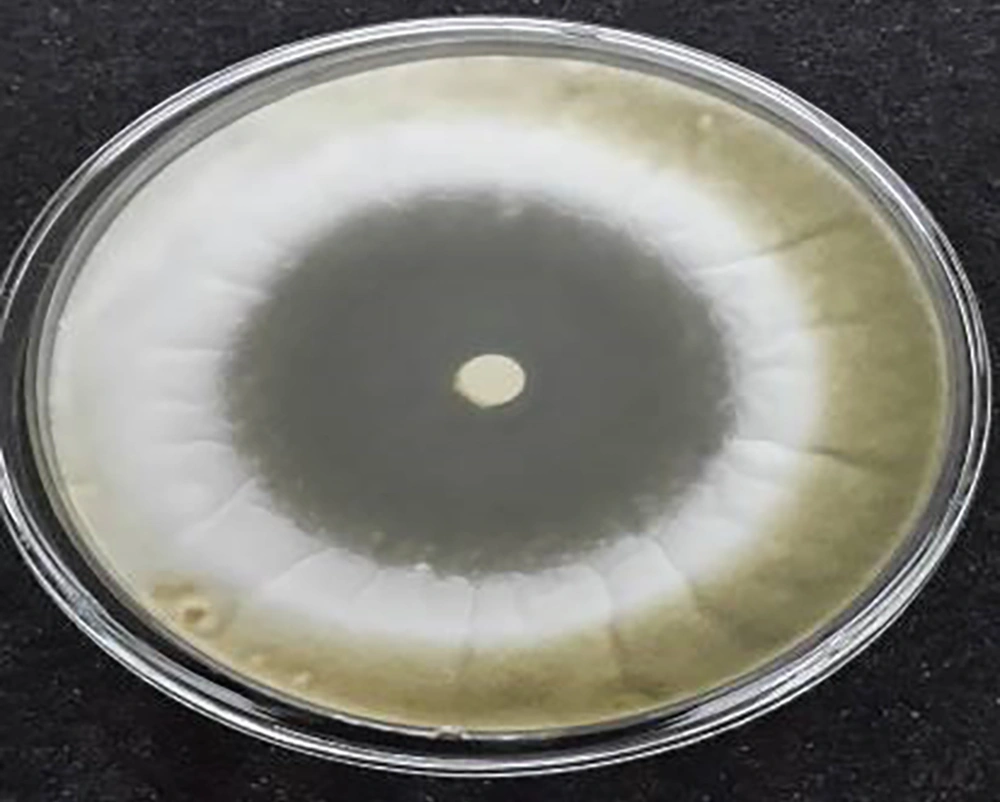
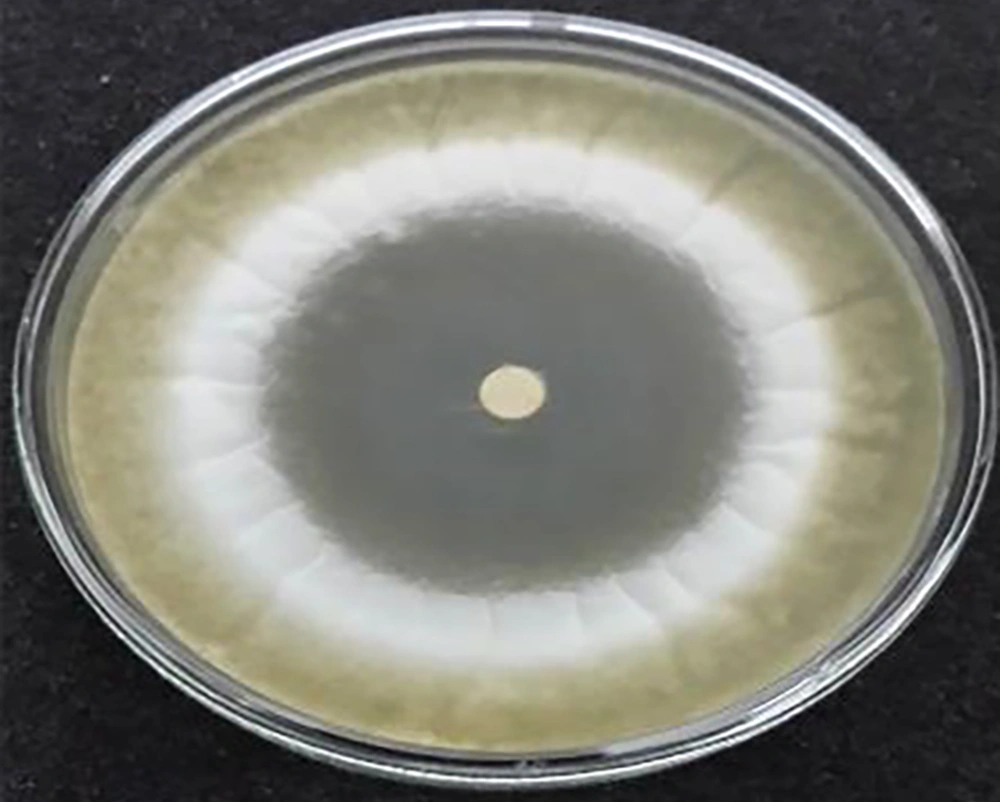
The against Aspergillus flavus PICC-AF39, and Aspergillus parasiticus NRRL2999 at concentrations 105 and 107 antifungal activities of the S. rechingeri essential oil were examined according to the inhibition zone diameter (Tables 1 - 4). Inhibition at a concentration of 20 µL S. rechingeri and inhibition at a concentration of 10 µL S. rechingeri Aspergillus parasiticus NRRL2999 are shown in Figures 1 and 2 respectively. The MIC and MFC numbers (Tables 5 and 6). Significant antifungal activity against the two fungal species tested was shown. The high diameters of growth inhibition was exhibited in two fungal species (76, 65 and 70, 55 mm) respectively for concentrations of 105 and 107. After three days of incubation, per petri plate even the dose of 1 μL of the oil on per disc, the S. rechingeri essential oil was effective against Aspergillus flavus PICC-AF39 and Aspergillus parasiticus NRRL-2999 with diameters of inhibition of almost 33, 25 and 18, 25 mm, respectively for concentrations 105 and 107. In the end of the tenth day, the diameter of inhibition zone was almost 18, 24, and 13, 23 mm for Aspergillus flavus PICC-AF39 and Aspergillus parasiticus NRRL2999, at concentration 105 and 107 respectively.
| Days | Aspergillus parasiticus (Colony Diameter in mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 µL | 10 µL | 5 µL | 4 µL | 3 µL | 2 µL | 1 µL | C | |
| 1 | 65 ± 0.00 | 54 ± 0.00 | 42 ± 0.00 | 40 ± 0.00 | 34 ± 0.00 | 29 ± 0.00 | 26 ± 0.00 | - |
| 2 | 65 ± 0.00 | 52 ± 0.00 | 36 ± 0.05 | 40 ± 0.06 | 30 ± 0.05 | 28 ± 0.05 | 26 ± 0.07 | - |
| 3 | 65 ± 0.00 | 52 ± 0.00 | 35 ± 0.04 | 36 ± 0.06 | 30 ± 0.04 | 28 ± 0.02 | 25 ± 0.02 | - |
| 4 | 58 ± 0.00 | 52 ± 0.00 | 35 ± 0.00 | 35 ± 0.03 | 30 ± 0.04 | 28 ± 0.02 | 25 ± 0.02 | - |
| 5 | 55 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.02 | 30 ± 0.02 | 28 ± 0.02 | 24 ± 0.02 | - |
| 6 | 40 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.03 | 30 ± 0.03 | 28 ± 0.00 | 24 ± 0.00 | - |
| 7 | 40 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.00 | 30 ± 0.03 | 28 ± 0.00 | 24 ± 0.00 | - |
| 8 | 40 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.00 | 30 ± 0.00 | 28 ± 0.00 | 24 ± 0.00 | - |
| 9 | 40 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.00 | 30 ± 0.00 | 28 ± 0.00 | 24 ± 0.00 | - |
| 10 | 40 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 34 ± 0.00 | 30 ± 0.00 | 28 ± 0.00 | 24 ± 0.00 | - |
| Days | Aspergillus parasiticus (Colony Diameter in mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 µL | 10 µL | 5 µL | 4 µL | 3 µL | 2 µL | 1 µL | C | |
| 1 | 76 ± 0.00 | 74 ± 0.00 | 67 ± 0.00 | 65 ± 0.00 | 65 ± 0.00 | 62 ± 0.02 | 60 ± 0.03 | - |
| 2 | 75 ± 0.00 | 69 ± 0.00 | 65 ± 0.00 | 62 ± 0.02 | 64 ± 0.04 | 61 ± 0.01 | 34 ± 0.06 | - |
| 3 | 75 ± 0.00 | 69 ± 0.00 | 65 ± 0.00 | 62 ± 0.02 | 61 ± 0.04 | 58 ± 0.04 | 33 ± 0.02 | - |
| 4 | 75 ± 0.00 | 69 ± 0.00 | 44 ± 0.07 | 62 ± 0.04 | 61 ± 0.04 | 46 ± 0.04 | 27 ± 0.06 | - |
| 5 | 72 ± 0.00 | 69 ± 0.00 | 44 ± 0.07 | 55 ± 0.06 | 41 ± 0.06 | 36 ± 0.04 | 18 ± 0.07 | - |
| 6 | 72 ± 0.00 | 67 ± 0.00 | 44 ± 0.07 | 50 ± 0.06 | 41 ± 0.06 | 36 ± 0.02 | 18 ± 0.07 | - |
| 7 | 72 ± 0.00 | 67 ± 0.00 | 44 ± 0.07 | 40 ± 0.07 | 38 ± 0.06 | 36 ± 0.06 | 18 ± 0.02 | - |
| 8 | 72 ± 0.00 | 67 ± 0.00 | 44 ± 0.05 | 40 ± 0.07 | 38 ± 0.01 | 36 ± 0.06 | 18 ± 0.02 | - |
| 9 | 72 ± 0.00 | 67 ± 0.00 | 44 ± 0.05 | 39 ± 0.02 | 38 ± 0.01 | 36 ± 0.06 | 18 ± 0.06 | - |
| 10 | 72 ± 0.00 | 67 ± 0.00 | 44 ± 0.05 | 39 ± 0.04 | 38 ± 0.01 | 36 ± 0.02 | 18 ± 0.02 | - |
| Days | Aspergillus parasiticus (Colony Diameter in mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 µL | 10 µL | 5 µL | 4 µL | 3 µL | 2 µL | 1 µL | C | |
| 1 | 55 ± 0.00 | 50 ± 0.00 | 40 ± 0.00 | 36 ± 0.06 | 31 ± 0.06 | 29 ± 0.06 | 25 ± 0.06 | - |
| 2 | 53 ± 0.00 | 50 ± 0.00 | 35 ± 0.00 | 31 ± 0.07 | 29 ± 0.07 | 27 ± 0.06 | 25 ± 0.00 | - |
| 3 | 53 ± 0.00 | 49 ± 0.00 | 35 ± 0.00 | 31 ± 0.01 | 29 ± 0.00 | 26 ± 0.01 | 25 ± 0.06 | - |
| 4 | 53 ± 0.00 | 49 ± 0.01 | 34 ± 0.00 | 30 ± 0.07 | 29 ± 0.07 | 26 ± 0.00 | 25 ± 0.06 | - |
| 5 | 53 ± 0.07 | 48 ± 0.00 | 33 ± 0.07 | 30 ± 0.00 | 28 ± 0.00 | 26 ± 0.06 | 25 ± 0.00 | - |
| 6 | 53 ± 0.00 | 48 ± 0.01 | 30 ± 0.07 | 30 ± 0.00 | 27 ± 0.07 | 26 ± 0.01 | 23 ± 0.06 | - |
| 7 | 53 ± 0.07 | 48 ± 0.00 | 30 ± 0.06 | 30 ± 0.00 | 27 ± 0.07 | 26 ± 0.00 | 23 ± 0.00 | - |
| 8 | 53 ± 0.00 | 48 ± 0.07 | 30 ± 0.00 | 30 ± 0.01 | 27 ± 0.00 | 26 ± 0.00 | 23 ± 0.00 | - |
| 9 | 53 ± 0.07 | 48 ± 0.00 | 30 ± 0.00 | 30 ± 0.00 | 27 ± 0.00 | 26 ± 0.06 | 23 ± 0.06 | - |
| 10 | 53 ± 0.07 | 48 ± 0.02 | 30 ± 0.06 | 30 ± 0.06 | 27 ± 0.00 | 26 ± 0.06 | 23 ± 0.01 | - |
| Days | Aspergillus parasiticus (Colony Diameter in mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 µL | 10 µL | 5 µL | 4 µL | 3 µL | 2 µL | 1 µL | C | |
| 1 | 70 ± 0.00 | 60 ± 0.00 | 56 ± 0.07 | 48 ± 0.00 | 40 ± 0.06 | 37 ± 0.06 | 37 ± 0.07 | - |
| 2 | 67 ± 0.00 | 60 ± 0.00 | 54 ± 0.07 | 48 ± 0.00 | 40 ± 0.06 | 37 ± 0.06 | 20 ± 0.06 | - |
| 3 | 67 ± 0.00 | 60 ± 0.00 | 54 ± 0.06 | 44 ± 0.00 | 40 ± 0.07 | 28 ± 0.06 | 18 ± 0.06 | - |
| 4 | 56 ± 0.00 | 52 ± 0.00 | 50 ± 0.07 | 43 ± 0.00 | 34 ± 0.00 | 28 ± 0.01 | 13 ± 0.07 | - |
| 5 | 56 ± 0.00 | 52 ± 0.01 | 47 ± 0.00 | 38 ± 0.07 | 34 ± 0.00 | 28 ± 0.01 | 13 ± 0.06 | - |
| 6 | 55 ± 0.00 | 52 ± 0.07 | 38 ± 0.00 | 30 ± 0.01 | 34 ± 0.00 | 28 ± 0.06 | 13 ± 0.06 | - |
| 7 | 55 ± 0.00 | 52 ± 0.07 | 38 ± 0.00 | 30 ± 0.07 | 34 ± 0.00 | 28 ± 0.06 | 13 ± 0.07 | - |
| 8 | 55 ± 0.00 | 52 ± 0.07 | 34 ± 0.07 | 30 ± 0.07 | 29 ± 0.07 | 28 ± 0.00 | 13 ± 0.07 | - |
| 9 | 55 ± 0.06 | 52 ± 0.06 | 34 ± 0.06 | 30 ± 0.06 | 29 ± 0.06 | 28 ± 0.00 | 13 ± 0.06 | - |
| 10 | 55 ± 0.06 | 52 ± 0.07 | 34 ± 0.07 | 30 ± 0.06 | 29 ± 0.06 | 26 ± 0.01 | 13 ± 0.06 | - |
| Satureja rechingeri | Values, ppm |
|---|---|
| Aspergillus parasiticus; NRRL2999; a concentration of 105 | |
| MIC | 50 |
| MFC | 200 |
| Aspergillus parasiticus; NRRL2999; a concentration of 107 | |
| MIC | 150 |
| MFC | 450 |
| Satureja rechingeri | Values, ppm |
|---|---|
| Aspergillus flavus; PICC-AF39; a concentration of 105 | |
| MIC | 50 |
| MFC | 100 |
| Aspergillus flavus; PICC-AF39; a concentration of 107 | |
| MIC | 150 |
| MFC | 300 |
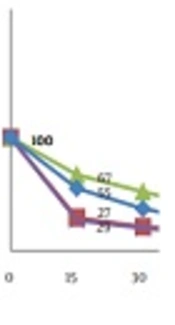
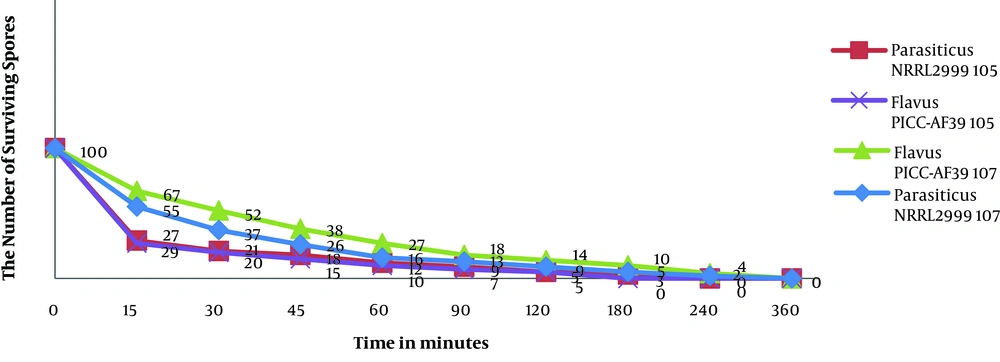
Oil killed more than 96% and 95% of the spores in both concentrations 105 and 107Aspergillus flavus PICC-AF39 tested within 3 hours also more than 97% and 98% of the spores in both concentrations 105 and 107Aspergillus parasiticus NRRL2999 within 4 hours tested. In both concentrations 105 and 107, 100% lethality was observed until 4 to 6 hours of the exposure to the oil. In Figure 3 it can be seen that the spore death happened in the early hours of the start of the experiment.
In conclusion, this study showed that S. rechingeri oil has a powerful antifungal activity against Aspergillus flavus and Aspergillus parasiticus. Also this essential oil can be used as a possible source of environmentally friendly herbs or botanical fungicides.
5. Discussion
Multiple technologies have been used to alleviate aflatoxin risk and remove fungus. Field management methods that decrease performance may also remove fungus and prevent aflatoxin. A variety of methods to remove and reduce aflatoxin such as physical procedures including heating, γ rays and extreme ultraviolet radiation, chemical procedures such as the utilization of chlorine, hydrogen peroxide, sodium sulphate, ozone gas, ammonia solution, alkali, biologic factors and mechanical procedure and other options to control the toxin. Natural products and aromatic organic compounds can be successful alternatives to chemical and physical factors and provide procedures to replace protection for agricultural products from toxigenic fungus such as Aspergillus flavus and Aspergillus parasiticus.
Since Aspergillus flavus and Aspergillus parasiticus ability to grow fast on food products that could damage the food industry, health, and economy. Therefore inhibiting the growth of this fungus can be helpful to the health of the community.
The results of this study can provide basic information on the efficiency and effectiveness of essential oil of S. rechingeri in reduction of the growth of fungi and antifungal activity of this plant in the country. The results in culture (in vitro) showed that the essential oil of S. rechingerihad antifungal activity against Aspergillus flavus and Aspergillus parasiticus are strong. In this study, the MIC for two concentrations of 105 and 107 of fungi Aspergillus flavus and Aspergillus parasiticus, 50, 50 and 100, 150 ppm by essential oil, respectively, of S. rechingeri. Satureja khuzestanicais research on the species and antifungal properties are demonstrated (11).
Other research has reported that the activity of thyme and Satureja in inhibiting the growth of fungi contaminating food crops and gardens and agricultural products in the world, capable of replacing anti-fungal chemicals are present (12).
A study on the inhibitory effect on the growth and aflatoxin production of Aspergillus parasiticus by S. hortensis essential oil had reached the conclusion that Satureja essential oil is an important potential for aflatoxin production inhibitor G1 and B1 are produced by Aspergillus parasiticus (13). Other results suggest the possibility of using other plant oils in controlling the growth of the fungus (14).
In the essential oil S. rechingeri had good antifungal activity against the two fungi species tested. This study indicates that S. rechingeri has considerable anti-Aspergillus activity at compared with C. Cyminum that MIC and MFC were 750, 1000 ppm and 3000, 2500 ppm, respectively (10).
H. suaveolens leaves essential oil showed activity against Aspergilus parasiticus that MIC and MFC were 40 and 80 µL/mL, respectively. Malele et al. (15) using the oils of Ocimum gratissimum and Thymus vulgaris reported complete inhibition of Aspergillus flavus and Aspergillus fumigatus at 800, 1000 respectively. Fungicidal kinetics of essential oils showed more than 50% spore death in 15 minutes (16) that at compared with the present study, while similar times have been reported.
5.1. Conclusions
Natural compounds such S. rechingeri for prevention of aflatoxinogen fungal growth are recommended because of strong antifungal activities.