1. Background
Methotrexate (MTX), which has long been used as an effective drug for treatment of numerous diseases such as rheumatoid arthritis, systemic lupus erythematosus, cancer, etc. (1), as many other drugs has some life-threatening adverse effects particularly liver enzyme abnormalities, hepatotoxicity, pneumonitis, and nephrotoxicity, which mostly limit its clinical application (2). Among these unpleasant effects, hepatotoxicity is more closely associated with the long term use of this drug. Although the underling mechanism is not fully understood, MTX causes a reduction in the availability of NADPH in cells and glutathione (GSH) level, which is dependent on NADPH to maintain its reduced state. All these can result in the inhibition of antioxidant defense system and increased oxidative stress (3, 4). Therefore, the necessity of antioxidant consumption during MTX treatment appears to be obvious (5).
The therapeutic evaluation of natural products in hepatic disorders has been noticed in last few years (6) and various natural and synthetic products have been tested by researchers to decrease hepatic damages (7, 8). Pomegranate (Punica granatum L.) has recently attracted all attention due to its beneficial and nutraceutical properties in several diseases such as cancer, diabetes mellitus, and cardiovascular diseases (9). This great action of pomegranate is ascribed to a large number of phytochemicals therein including hydrolysable tannins and phenolic compounds found in different parts of this miracle fruit (9, 10). The most interesting thing in this regard is the higher amount of these phytochemicals in non-edible fraction especially in the peel of the fruit. Ellagitannins and the pomegranate-peculiar punicalagin are important polyphenols, which function as pivotal anti-inflammatory and antioxidant agents (9). Based on the findings of latest researches on pomegranate peel extract (PPME), it seems that this waste part of the fruit might play a more important and promising role in the control of oxidative stress through radical scavenging activity and chelation of transition metals (9). There are several in-vitro and in-vivo evidence and epidemiological studies confirming antioxidant property of PPME and its efficacy in the control of oxidative stress-mediated diseases (11).
Although the use of extracts isolated from pomegranate peels has been recently the center of attention because of their potential function in different pathways, research in this regard is scanty that does not provide information about its efficacy in the improvement of antioxidant enzymes related to specific tissues including liver after methotrexate administration.
2. Objectives
The current study was undertaken to investigate the effects of Methotrexate and pomegranate peel methanolic extract (PPME), alone and in combination, on liver antioxidants of rats.
3. Methods
3.1. Extraction
Pomegranates (Punica granatum L., Post-Ghermez variety, 5-64-WS) (12) were purchased from the Shiraz suburbs (Fars province, Iran). The pomegranates were washed and peeled manually and the peels were separated and air dried in a 40°C oven for 24 hours. The peels turned into a coarse powder. After that, the powder (500 g) was mixed with methanol (Merck, Germany) (1:10 w/v). After 96 hours, the mixture was filtered through 0.45 µ pore size filter (13, 14) and the solvent was completely evaporated by a rotary vacuum evaporator (Hidolf, Germany) at 40°C. The value of extraction efficiency was about 5% and the pomegranate peel extract (PPME) was stored at -70°C in a freezer until performing the tests.
3.2. Animals
32 male Wistar rats, weighing 200 ± 15 g, were maintained under standard conditions (22 ± 2°C, 12 hours light/12 hours dark cycles) during the experiments. A standard diet and clean water were provided for the rats ad libitum.
The proposal and the animal procedure were approved by the Tabriz University of Medical Sciences’ research ethics committee (certificate No.: 5-4-11060, 24 Feb 2014) and the study was conducted according to the European commission directive 86/609/EEC for animal experiments.
3.3. Procedures
Similar previous animal studies were considered for choosing sample size (15). The rats were randomly divided into 4 groups using the Rand-list software (n = 8). Group I was the placebo control group that orally received normal saline for 18 days. Group II was the pomegranate group that orally received 500 mg/kg PPME for 18 days. Group III was the MTX group that intramuscularly received 10 mg/kg MTX for 3 days, beginning from the 10th day. Group IV was a treatment group that orally received 500 mg/kg PPME for 18 days, and intramuscularly received 10 mg/kg MTX for 3 days, beginning from the 10th day.
After the intervention, the rats were euthanatized. The liver tissue samples were obtained. 100 mg samples of the tissue were homogenized by a glass tissue homogenizer in 1 mL ice cold PBS (pH, 7.4). Afterwards, the homogenized samples were centrifuged at 10,000 g for 10 minutes at 4°C. The supernatants were removed and placed in -70°C deep freezer until performing the tests.
3.4. Biochemical Analyses
Total antioxidant capacity (TAC), glutathione peroxidase (GPx), and superoxide dismutase (SOD) of the supernatants were assayed by Biorex diagnostics commercial kits (UK). Malondialdehyde (MDA) contents of the supernatants were determined using thiobarbituric acid method (16). Catalase enzyme activities of the supernatants were determined by Cayman kit (USA). The protein contents of the supernatants were assessed by Parsazmun kit (Karaj, Iran). All results were normalized by the protein contents of supernatants. An Abbott Alcyon 300 automated biochemistry analyzer (USA) was used for the abovementioned colorimetric assays.
3.5. PPME Analyses
The PPME antioxidant capacity was assayed by the DPPH assay method (17). The samples reduction capacities 50 (RC50) were stated as µg/mL. The quercetin RC50, as the control material, was 3.9 µg/mL. Total phenolic equivalent was assessed by Folin-Ciocalteu reagent, while a Gallic acid standard curve was used. The result reported as mg of Gallic acid equivalent (GAE) per gram of extract (18). Total flavonoids were assayed by spectrophotometric method (19).
3.6. Statistical Analysis
SPSS (version 11.5) was used for statistical analyses (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to compare parameters between the groups for normally distributed data. Tukey’s post-hoc test was used for multiple comparisons. Furthermore, the results of Levene’s test showed the equality of variances of the groups. The results were expressed as mean ± 1 standard deviation (SD). P values less than 0.05 were considered statistically significant.
4. Results
4.1. The Antioxidant Properties, Polyphenol and Flavonoid Contents of PPME
The composition of the pomegranate peel methanolic extract in terms of poly-phenolic and flavonoid compounds and antioxidant properties is represented in Table 1.
| Sample | Antioxidant Activity, RC50; µg/mL | Total Phenolic Content, mg GAE/g Extract | Total Flavonoid, % |
|---|---|---|---|
| Pomegranate Peel Extract | 15 ± 0.2 | 153.2 ± 0.3 | 1.04 ± 0.03 |
4.2. Changes in Antioxidant Enzymes Following MTX and PPME Administrations
Table 2 displays the status of all parameters measured in this research in the 4 study groups. MDA and surprisingly SOD and TAC liver contents showed a great enhancement while a reduction was observed in the content of liver GPX and CAT in the group of rats receiving MTX compared to the other groups. PPME administration with MTX augmented the levels of GPx and CAT and decreased the contents of MDA, SOD, and TAC compared to the MTX-treated group.
| Group | GPx | MDA | CAT | SOD | TAC |
|---|---|---|---|---|---|
| Control | 0.427 ± 0.037 | 0.533 ± 0.028 | 25.615 ± 1.397 | 5.180 ± 1.007 | 0.197 ± 0.005 |
| PPME | 0.298 ± 0.058b | 0.528 ± 0.054 | 28.516 ± 8.707 | 4.434 ± 0.806 | 0.194 ± 0.005 |
| MTX | 0.260 ± 0.113 | 1.698 ± 0.400b | 18.828 ± 2.323b | 8.204 ± 2.315c | 0.216 ± 0.016 |
| MTX + PPME | 0.296 ± 0.052b | 1.735 ± 0.693b | 26.588 ± 10.035 | 7.371 ± 1.739c | 0.210 ± 0.219 |
Abbreviations: CAT (nmol/min/mg protein), Catalase; GPx (u/mg protein), Glutathione peroxidase; MDA (nmol/mg protein), Malondialdehyde; SOD (u/mg protein), Superoxide dismutase; TAC (mmol Trolox equivalent/mg protein) total antioxidant capacity.
aValues are expressed as mean ± SD.
bSignificantly different at (P < 0.05) when compared with the control group.
cSignificantly different at (P < 0.05) when compared with PPME-treated rats.
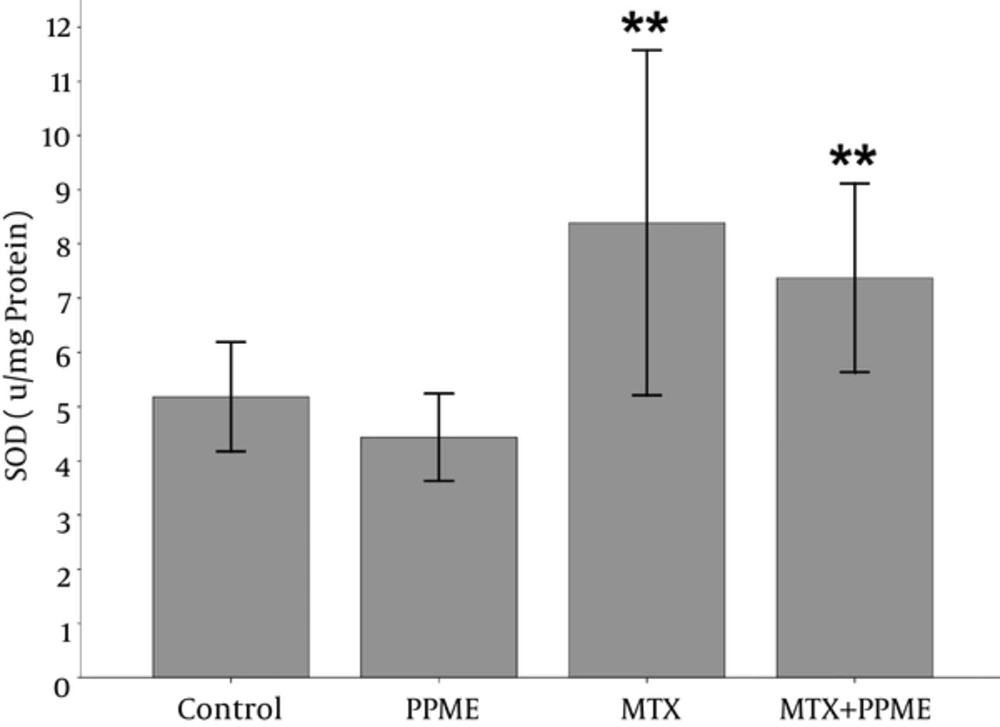
4.3. Effects on SOD
Treatment of the rats with MTX surprisingly caused a significant increase in the SOD liver content when compared with the group receiving PPME (P = 0.01). In addition, MTX combined with PPME pre-treatment led to a significant increase in the liver SOD content in comparison with the group receiving PPME alone (P = 0.039) (Figure 1).
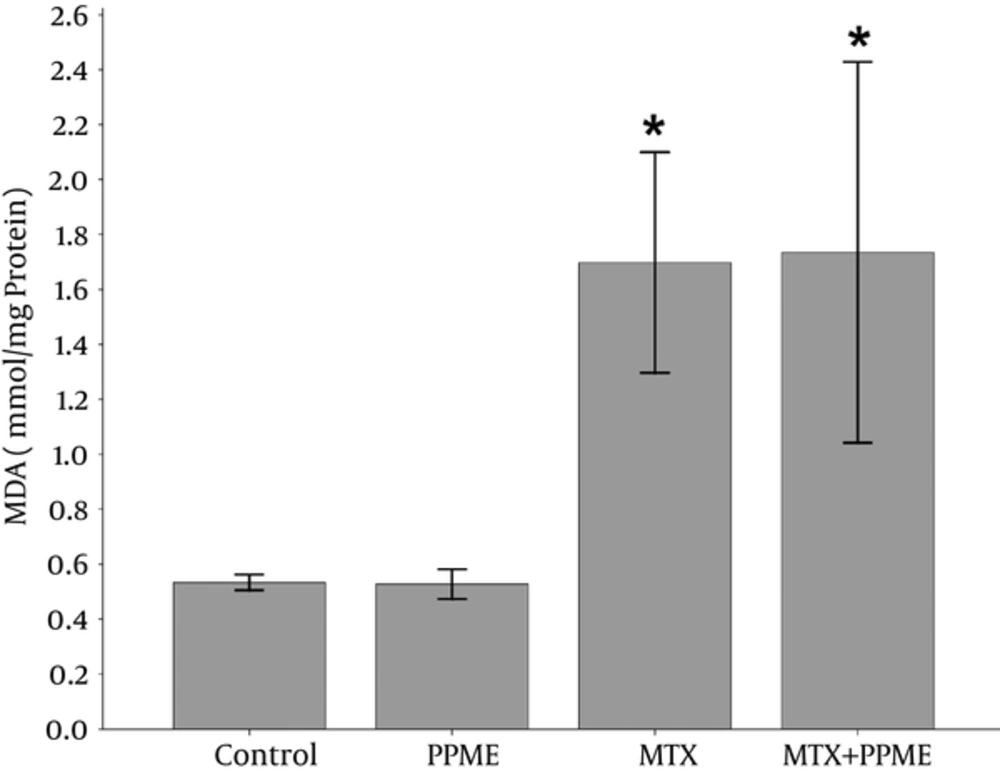
4.4. Effects on MDA
The content of liver MDA as a product of lipid peroxidation increased significantly following MTX administration when compared with the control (P = 0.036) and PPME receiving groups (P = 0.008).
However, the administration of both PPME and MTX did not alter the MDA level compared to the MTX-treated group (Figure 2).
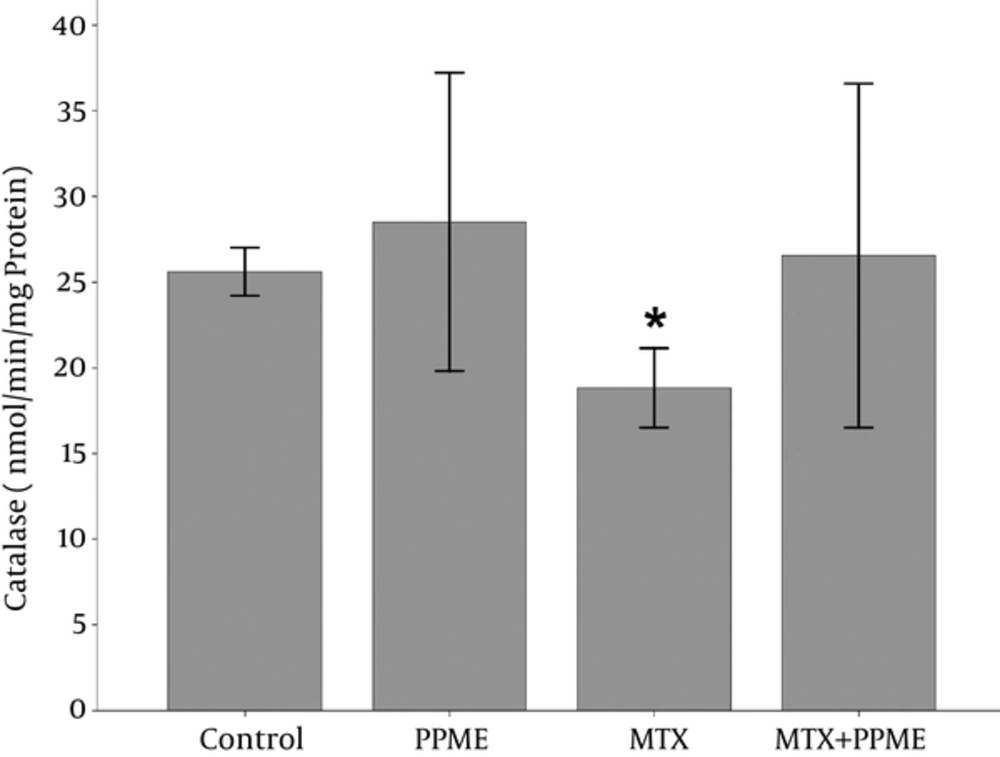
4.5. Effects on CAT
The administration of MTX caused a reduction in the content of liver CAT compared to the control group (P = 0.016). It increased non-significantly following the administration of PPME combined with MTX when compared with the MTX-treated group (Figure 3).
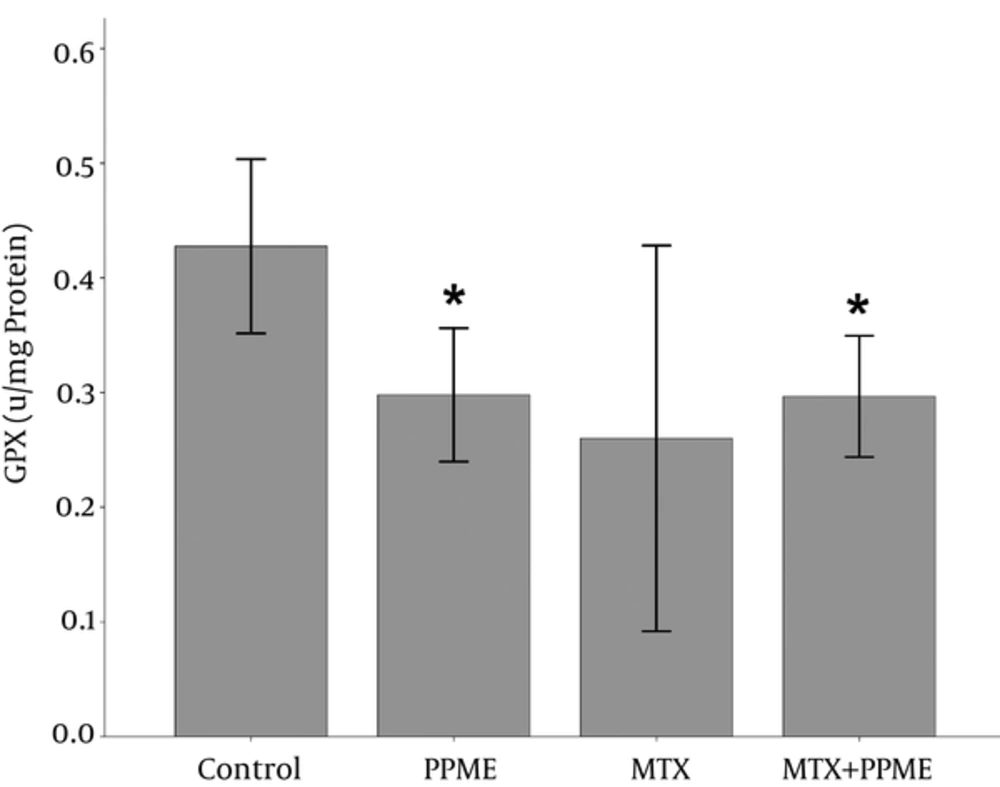
4.6. Effects on GPx
The PPME administration caused a significant increase in the content of liver GPx when compared to the control group (P = 0.032) and PPME combined with MTX-pretreated group (P = 0.019). However, the low content of liver GPx in the MTX-treated rats compared to the other groups was not significantly different (Figure 4).
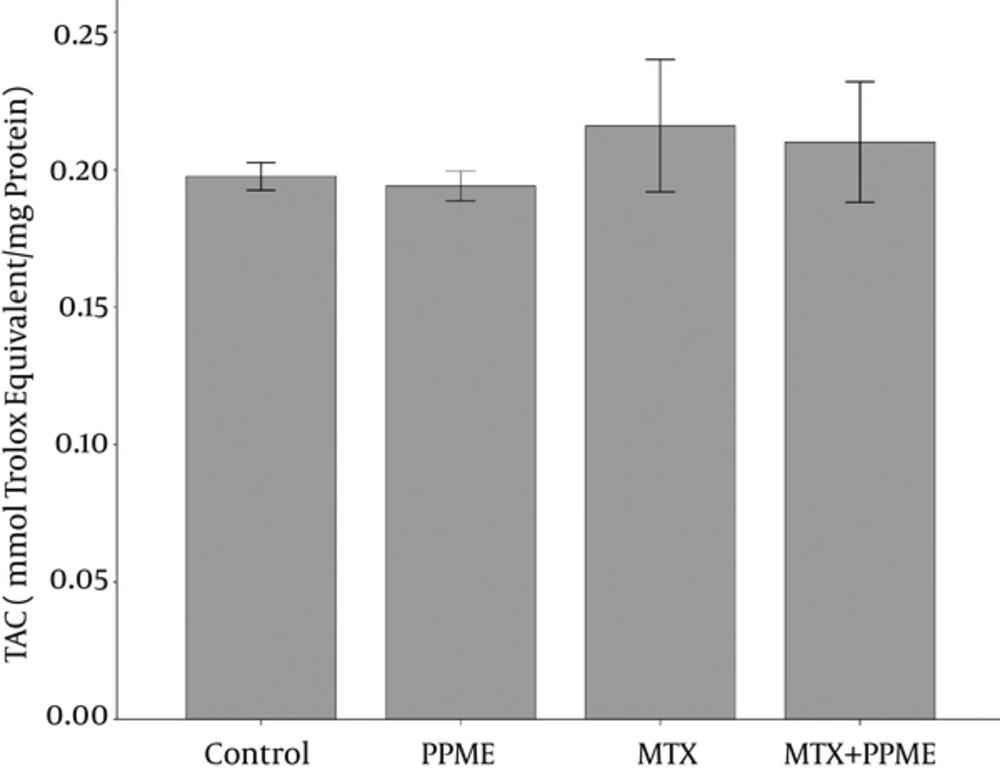
4.7. Effects on TAC
TAC measurements in the 4 groups are represented in Figure 5. TAC depletion (0.194 ± 0.005 nmol/mg protein) was found in the rats receiving PPME when compared to the other groups, though the difference was not significant. MTX administration augmented TAC level as compared to the other groups (P > 0.05).
5. Discussion
Composition of the pomegranate peel methanolic extract was similar to that of the polyphenol extractives in a research conducted by Saad et al. Similarly, on the basis of their findings, phenolic contents of pomegranate peel ranged from 134.3 to 181.0 mg GAE/g DW [mg Gallic acid equivalent (GAE) per g of dry weight (DW)] (20).
Another study found that water extract from fresh peel contained higher levels of polyphenol contents such as 223 ± 3 mg GAE/100 g fresh peel (8) and 344 - 381 mg GAE/100 g fresh peel (21). Given the higher polyphenol contents in unwashed peels, it seems that water-soluble polyphenols might be eliminated by washing (20). Some conflicting data in this regard could be explained by difference in the stage of maturity and the mean annual precipitation or even in the solvents used for extraction (20). Negi et al. found that among methanol, acetone, and water used for the extraction of antioxidants from pomegranate peel, methanol extract represented the maximum antioxidant activity (22). A high extent of pomegranate varieties exists in Iran; the extract composition is affected by the pomegranate genotype diversity, which is classified on the basis of fruit characteristics as morphology, taste, color, size, soluble solids, acidity ratio, time of ripening, and different geographical regions (12).
The present study showed an impaired redox status following MTX administration. MTX-treated rats indicated decreased levels of antioxidant enzymes including GPx and CAT. On the other hand, MDA as a byproduct of lipid peroxidation elevated in the rats receiving MTX as compared to the other groups. Additionally, an improved antioxidant system after PPME administration alone or combined with MTX was observed. Despite these effects, no significant differences were observed between the group receiving MTX and the group receiving PPME and MTX combination.
MTX can adversely affect different tissues especially liver, which can be damaged histopathologically and biochemically. It is postulated that most of the MTX metabolisms like converting to its active metabolite, 7-hydroxymethotrexate, occur in the liver. Then, it can interact with some cellular pathways to, for example, inhibit oxygen uptake in some cells, reduce the trans-plasma membrane redox activity, and increase glycogenolysis. All these events result in the inhibition of energy production, which can be compensated with some processes in mitochondria generating ROS as byproducts. On the other hand, cellular antioxidant defense system, which is responsible for scavenging activity, is weakened consequently due to the toxicity of MTX (3). In this sense, the inhibition of NADPH-dependent dehydrogenases such as glucose-6-phosphate dehydrogenase, which has been underlined in previous studies (23), makes us think that MTX might be involved in the reduction of NADPH availability in the cells. This event consequently causes an inhibition of glutathione reductase activity, which is dependent on NADPH and eventually the inhibition of GSH cycle (24). Therefore, this conclusion can be drawn that NADPH reduction accounts for the impaired antioxidant system following MTX administration. Our findings were in line with several recent evidence on the unpleasant effects of MTX suggesting the reduction of antioxidant liver content such as GPx and CAT and the increase of MDA level as a pro-oxidant agent (4, 25-27).
Our study has yielded striking results, as well. On the basis of our findings, exposure to MTX caused an increase in TAC as well as SOD levels. The findings of the current study are consistent with those of Oktar and colleagues who found an enhancement in the TAC level of liver in MTX-treated mice. They showed significantly higher levels of total antioxidant capacity (TAC) and total oxidative stress in the liver after methotrexate administration compared to the control group (28). Another unanticipated finding was the higher levels of SOD in the MTX group, which is in agreement with the findings of Najah et al. showing an elevated content of liver SOD in MTX-treated rabbits (29) and another study in which CAT levels markedly increased in the liver of rats after MTX administration (30).
More recently, literature has suggested the protective effects of pomegranate peel extract on food oxidative stability after addition to some food items including dairy products, fish, wheat bread, de-alcoholised red wine, jam, and juice without any unpleasant effect on taste or other aspects of their health properties (9). Additionally, most evidence collected to date has supported its antioxidant feature in different tissues (8, 31-34).
The outputs of this study are in line with other researches supporting the effects of pomegranate peel extract on antioxidant enzymes in rats’ liver (31, 32). In our study, PPME administration either alone or in combination with methotrexate caused a great enhancement in the levels of some antioxidant enzymes such as GPx and CAT and a reduction in the MDA level when it was used alone. However, SOD and TAC levels which surprisingly elevated in the MTX group tended to decrease in the PPME receiving group and the MTX receiving combined with PPME pretreatment group, as well. This finding is inconsistent with those showing an enhancement in the plasma level of TAC after the administration of methanol extract of pomegranate peel against oxidative stress induced by treatment with mercuric chloride (HgCl2) in a rat model (33).
The reason for this is not clear; but it may have something to do with the solvent for extraction that can affect the polyphenols content in pomegranate peel or its unknown optimal dose. In addition, the dose of methotrexate used to induce oxidative stress may not be proper to change SOD and TAC liver content. The results would be more reliable if either there was a control group with no injection or the intervention period was longer or different doses of drug and extract were used. However, this is the first study designed to investigate the effects of PPME on liver antioxidant changes induced by methotrexate. The determination of poly-phenolic and flavonoid compounds and antioxidant properties in pomegranate peel methanolic extract was one of the strength of this work.
In conclusion, Methotrexate can significantly increase SOD and MDA and decrease CAT contents and, on the other hand, PPME can significantly decrease GPx and relatively prevent the effects of methotrexate on SOD and CAT contents of the liver tissue of rats.
Due to the controversies between the effects of methotrexate and PPME on different antioxidants as well as in different tissues, it seems that more research needs to be undertaken on this topic.