1. Background
Vernonia amygdalina L. (bitter leaf) is an evergreen plant that can grow to 2 - 5 m in height, belonging to Asteraceae family that consists of more than 1000 species. It is widely found in Africa (mostly West Africa) and Asia (1, 2). It grows mostly along drainage, natural forest, home gardens, and commercial plantations (3, 4). This plant is popularly consumed as soup in Nigeria (Onugbo), food supplement in Cameroon (Ndole), and soil fertility improver in Ethiopia (3, 5, 6). V. amygdalina leaf is rich in fatty acids such as palmitic, oleic, and linoleic acids (7, 8).
Studies on the isolation of bioactive compounds revealed the presence of steroidal glycosides, triterpenoids, flavonoids, and numerous classes of sesquiterpene lactones like vernolide, vernodalol, epiverdalol, and vernonioside A, B, C, D (6, 9-11). Due to the presence of these bioactive compounds, V. amygdalina leaf has been an indispensable plant, especially, in pharmaceutical and food industries. Several pharmacological effects such as antioxidant, anticancer, anti-inflammatory, antimalarial, antimicrobial, antidiabetes, and anti-leukemia, among others, have been reported for the extracts (6, 12, 13).
In the past years, several novel extraction methods such as microwave-assisted extraction (MAE), supercritical fluid extraction (SFE), ultrasound-assisted extraction (UAE), and pressurized solvent extraction (PSE) have been introduced and investigated, most of which were reported to improve the extract quality, efficiency, solvent consumption, and extraction time (14). MAE has been reported to possess some advantages over other extraction methods, most especially a significant reduction in processing time and a high yield of active extracts (15). This method is influenced by many factors, which include temperature, microwave power, irradiation time, solvent type, and feed-to-solvent ratio. Likewise, the mode of plant matrix drying can also affect the yields of the extracts and the antioxidant activity (16, 17).
2. Objectives
The aim of this study was to investigate the effect of MAE parameters on the extraction and total phenolic content yields and determine the IC50 antioxidant values of the drying method with the highest yields.
3. Methods
3.1. Materials and Reagents
The V. amygdalina leaves obtained from Gambang (latitude: 3°42’25.183”N and longitude: 103°6’8.982”E), Malaysia, were identified by Prof. Nour Hamid Abdurahman. The leaves were washed and dried under different drying conditions (shade, sun, and oven at 40, 50, and 60°C). The dried samples were blended (Panasonic Blender PANA-MX-801S HG, Malaysia) and sieved to 105 mm particle sizes. Ethanol, methanol, sodium carbonate anhydrous, 2, 2-diphenylpicrylhydrazyl (DPPH), ascorbic acid, potassium persulfate, 2, 2’-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS+•), and Folin-Ciocateu phenol reagent were purchased from Sigma-Aldrich (M) Sdn Bhd, Selangor. Distilled water was obtained from the Faculty of Chemical and Natural Resources Engineering laboratory. All the chemicals used were of analytical grade without further purification.
3.2. Microwave-Assisted Extraction of V. amygdalina Leaf
An ethos reflux microwave extractor (1000 W, frequency 2450 MHz, Milestone, Italy) was used for the extraction. The extraction process was done in an enclosed vessel and no evaporation was observed. Homogeneous powdered V. amygdalina leaf samples (10 g) were mixed with a measured quantity of extracting solvent (distilled water or ethanol). The mixture was loaded into the microwave extractor and the parameters were set. After the extraction process, the mixture was cooled to room temperature and filtered. Thereafter, the filtrate was concentrated until dryness using a rotary evaporator and stored at 4°C prior to further analysis.
3.3. Determination of Extraction Yield
The percentage yield of the extracts was determined based on the dry weight (d.w.) using Formula 1.
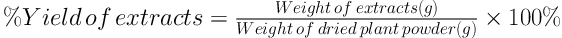
3.4. Total Phenolic Content Determination
The colorimetric assessment of total phenolic content was done based on the procedure described by Saravanan and Parimelazhagan (18) and Dahmoune et al. (19) with some modifications. In brief, the extracts were diluted to tenfold to fall within the optimal absorbance range (OD value 0.1 - 0.9). Gallic acid (50 - 500 mg/L) was used to construct a standard calibration curve. The extract of 100 µL was added to distilled water to make up 1 mL. Then, 0.2 mL of Folin-Ciocalteu phenol reagent was added. The mixture was thoroughly mixed and incubated for 5 min before 0.6 mL of 0.2 mM sodium carbonate solution was added. The mixture was incubated for 90 min at the room temperature in the dark and the absorbance of the mixture was measured at 765 nm against the blank (sample solvent) using a UV-vis Spectrophotometer (Hitachi U-1800, Japan). The analyses were performed in triplicate and the results were expressed in gallic acid equivalents (mg GAE/g d.w.). The total phenolic content of the extracts was calculated using Formula 2.
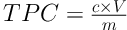
where c is the sample concentration obtained from the calibration curve (mg/L), V is the volume (mL) of the solvent used in the extraction, and m represents the weight (g) of the dried sample used.
3.5. Antioxidant Activity
3.5.1. DPPH Free Radical Scavenging Activity
The DPPH free radical scavenging assay of the V. amygdalina leaf extracts was done as described by Ayoola et al. (20) and Alam et al. (21) with some modifications. A 2.5 mL volume of 0.1 mM DPPH solution was added to 0.5 mL of the extract (at concentrations of 100, 200, 400, and 500 µg/mL) dissolved in analytical grade methanol. The absorbance was recorded at 517 nm using a UV-VIS Spectrophotometer (Hitachi U-1800, Japan) after 30 min of incubation at the room temperature in the dark. The analyses were repeated thrice. Methanol was used as the blank. The percentage of inhibition was calculated using Formula 3.
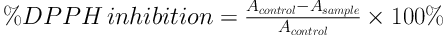
where Acontrol is the absorbance of the mixture of methanol and DPPH solution and Asample is the absorbance of the mixture of the sample extract and DPPH solution.
3.5.2. ABTS+• Antioxidant Activity
This assay was carried out following the procedure described by Thaipong et al. (22). Briefly, the ABTS radical cation was prepared by mixing 7 mM ABTS stock solution with 2.45 mM potassium persulfate in equal quantities. Then, the mixture was allowed to stand in the dark at the room temperature for 12 h until the reaction completed and the absorbance was stabled. The fresh working solution of ABTS radical cation was prepared by diluting 2 mL of ABTS+• solution with 120 mL methanol to obtain an absorbance value of 1.1 ± 0.02 at 734 nm using a UV-VIS Spectrophotometer (Hitachi U-1800, Japan). The V. amygdalina leaf extract of 0.15 mL (at concentrations of 100, 200, 400, and 500 µg/mL) was mixed with 2.85 mL of ABTS+• solution and allowed to stand in the dark for 2 h at the room temperature. Thereafter, the absorbance was measured at 734 nm using a UV-VIS Spectrophotometer (Hitachi U-1800, Japan). Methanol was used as the blank and Formula 4 was used for determining the percentage of inhibition.
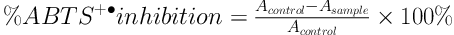
where Acontrol is the absorbance of the mixture of methanol and ABTS+• solution and Asample is the absorbance of the mixture of the sample extract and ABTS+• solution.
3.6. Statistical Analysis
The extraction runs and all the analyses were done in triplicate. The influence of each extraction parameter on the extract yield and total phenolic content yield in the one-factor-at-a-time experiments were expressed as mean ± SD. The One-way ANOVA was used to analyze the results statistically.
4. Results and Discussion
4.1. Effect of Irradiation Time on the Extraction and Total Phenolic Content Yields of V. amygdalina Leaves
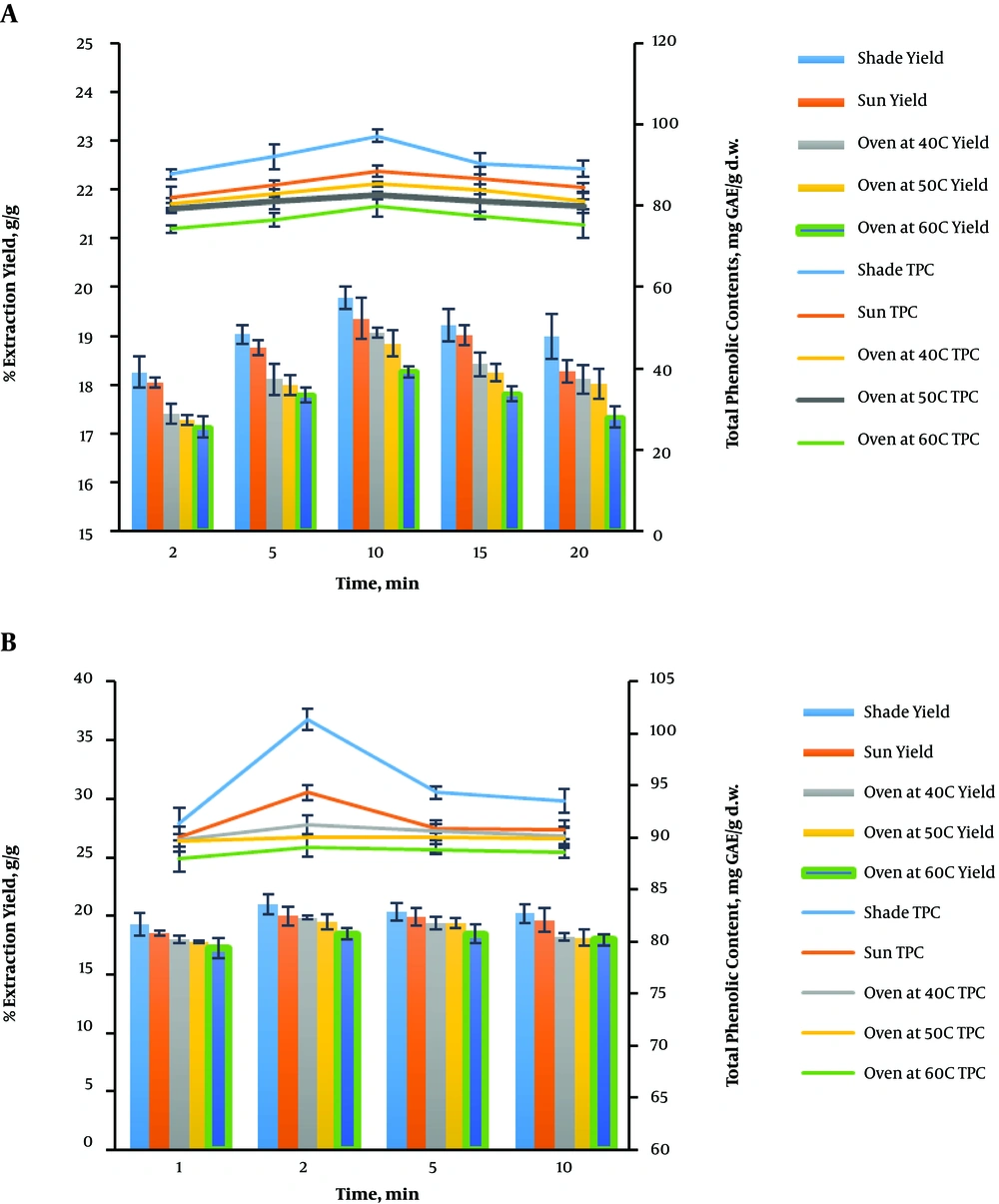
The extraction and total phenolic content yields at different irradiation times (2, 5, 10, 15, and 20 min), the microwave power of 400 W, the temperature of 100°C, and the feed-to-solvent ratio of 1:10 for different drying methods (shade, sun, oven drying at 40, 50, and 60°C) using water as the extracting solvent are shown in Figure 1A. The extraction and TPC yields were found to be in the range of 18.26% - 19.78% g/g and 87.74 - 97.15 mg GAE/g d.w.; 18.04% - 19.36% g/g and 82.11 - 88.43 mg GAE/g d.w.; 17.41% - 19.06% g/g and 80.47 - 85.31 mg GAE/g d.w.; 17.28% - 18.84% g/g and 79.44 - 82.65 mg GAE/g d.w.; and 17.13% - 18.27% g/g and 74.34 - 79.88 mg GAE/g d.w. for shade, sun, oven drying at 40, 50, and 60°C, respectively. It can be observed that the yields increased with time until 10 min where it began to decline gradually. Shade drying showed the best drying condition with the highest yields of extract (19.78 ± 0.23% g/g) and TPC (97.15 ± 1.52 mg GAE/g d.w.) when the irradiation time of 10 min was attained.
In the same vein, when ethanol was used as the extracting solvent, the extraction and total phenolic content yields ranged from 19.28% - 20.99% g/g and 91.35 - 101.35 mg GAE/g d.w.; 18.54% - 20.00% g/g and 90.11 - 94.34 mg GAE/g d.w.; 18.01% - 19.84% and 89.84 - 91.23 mg GAE/g d.w.; 17.79% - 19.52% g/g and 89.75 - 90.09 mg GAE/g d.w.; and 17.23% - 18.47% g/g and 87.97 - 89.11 mg GAE/g d.w. for shade, sun, oven drying at 40, 50, and 60°C, respectively (Figure 1B). The conditions for the extraction were irradiation time of 1 - 15 min, ethanol concentration of 20% v/v, microwave power of 300 W, the temperature of 70°C, and the feed-to-solvent ratio of 1:8. An increase in the irradiation time from 1 to 2 min increased the extraction and TPC yields; extraction reached its maximum at 2 min, and then the yields decreased with the extension in the irradiation time. The highest yields were obtained for the extraction using the shade drying condition.
In relation to the literature, the maximum yield of total polyphenols was attained at 4 min when caffeine was extracted from tea green leaves using MAE and ethanol as the extracting solvent (23). The highest extraction (22.48 ± 0.17%) and total polyphenols yields (5.54 ± 0.08) at 10 min were reported during the extraction of phenolics from oregano (24). More so, Okoduwa et al. reported that the highest extraction yield of 20.9 ± 1.05% was obtained from MAE at 39 min after the extraction of shade-dried V. amygdalina leaves using ethanol as the extracting solvent (25).
4.2. Effect of Microwave Power on the Extraction and Total Phenolic Content Yields of V. amygdalina Leaves
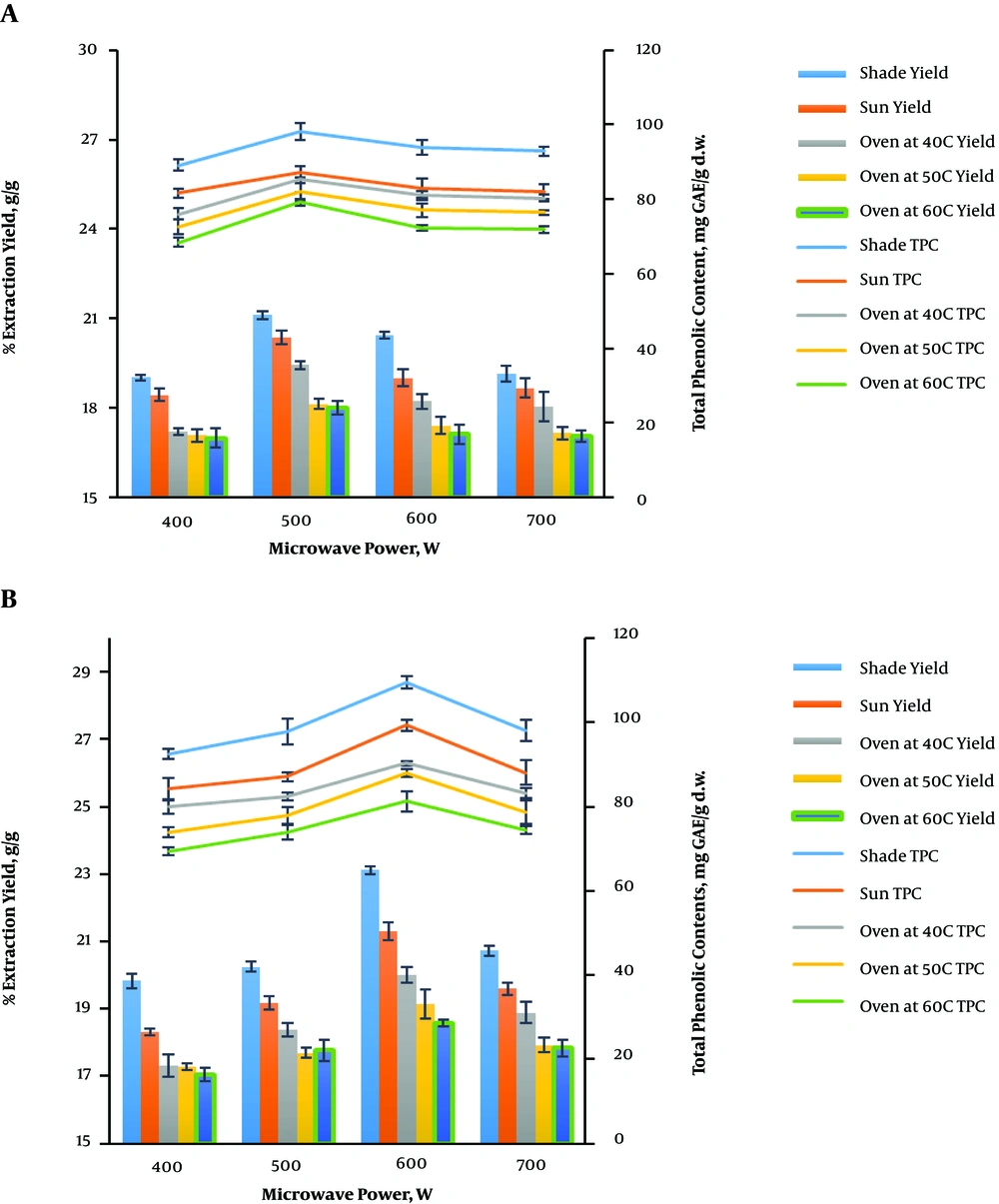
The experimental results for the extraction and total phenolic content yields from V. amygdalina leaves at different microwave power levels (400, 500, 600, and 700 W), the irradiation time of 10 min, the temperature of 100°C, and the feed-to-solvent ratio of 1:10 using water as the extracting solvent at different drying conditions are shown in Figure 2A. An increase in the microwave power from 400 to 500 W resulted in the yields improvement; the highest extraction and TPC yields from the extract were obtained at 500 W, and any further increment in the microwave power resulted in a decrease in the yields. This may be due to the degradation of phenolic acids at the higher wattage (24, 25). The highest extraction (21.12 ± 0.13% g/g) and TPC (98.25 ± 2.31 mg GAE/g d.w.) yields were obtained for shade drying at 500 W.
Figure 2B shows the microwave power variation (400, 500, 600, and 700 W) at a constant ethanol concentration of 20% v/v, the irradiation time of 2 min, the temperature of 70°C, and the feed-to-solvent ratio of 1:8. Ethanol was used as the extracting solvent. The extraction and total phenolic content yields were in the range of 19.83% - 23.11% g/g and 92.52 - 109.52 mg GAE/g d.w.; 18.32% - 21.30% g/g and 84.26 - 99.34 mg GAE/g d.w.; 17.31% - 20.01% g/g and 79.93 - 90.32 mg GAE/g d.w.; 17.27% - 19.14% g/g and 73.95 - 88.05 mg GAE/g d.w.; and 17.05% - 18.56% g/g and 69.42 - 81.28 mg GAE/g d.w. for shade, sun, oven drying at 40, 50, and 60°C, respectively. The highest extraction (23.11 ± 0.12% g/g) and total phenolic content (109.52 ± 1.52 mg GAE/g d.w.) yields were obtained at 600 W using the shade drying method. The accelerated extraction of V. amygdalina leaves can be related to the direct effects of microwave energy on phytomolecules through ionic conduction and dipole rotation, which resulted in power dissipation of the volumetric design in the plant materials and the solvent used, thus generating molecular movement and heating. Similar observations were reported for the extraction of bioactive flavonolignan (26).
4.3. Effect of Temperature on the Extraction and Total Phenolic Content Yields of V. amygdalina Leaves
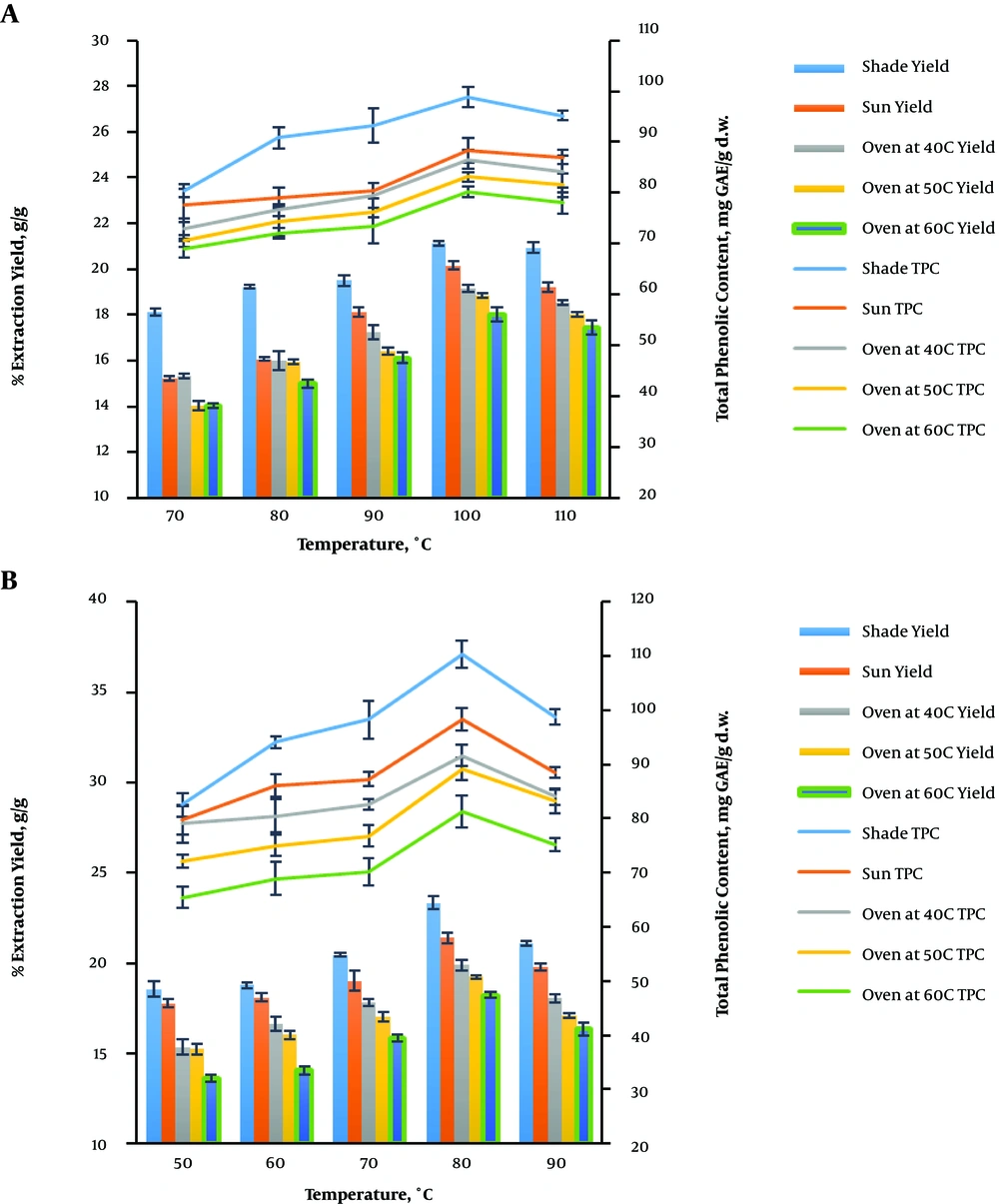
For the extraction of bioactive compounds from plant materials, the temperature is an indispensable factor that determines the extraction yields (27, 28). Figure 3A shows the effect of temperature on the extraction and total phenolic content yields of V. amygdalina leaves using MAE at different temperatures (70, 80, 90, 100, and 110°C) and a constant irradiation time of 10 min, the microwave power of 500 W, and the feed-to-solvent ratio of 1:10. Water was used as the extracting solvent. It can be seen that the yields significantly improved as the temperature raised from 70°C to 100°C but declined when the temperature was set to 110°C. An increase in the yields with a temperature increase until the boiling point of the solvent was in agreement with the previous studies (24, 26, 28). The highest values of extraction (2.12 ± 0.12% g/g) and total phenolic content (98.89 ± 2.01 mg GAE/g d.w.) yields were obtained at a temperature of 100°C using the shade drying method. Hithamani and Ramalakshmi reported similar results when extracting phenolics from Origanum vulgare using MAE whereby the maximum yield (22.48 ± 0.16%) and total polyphenols (5.29 ± 0.06 mg GAE/g d.w.) were obtained at 100°C (24). The recovery of phenolic compounds from the plant matrix could be enhanced by heating that improves the diffusivity of the extraction solvent into the plant matrix and then enhances the solubility of phenolic compounds in the extraction solvent (14, 19, 25, 28).
The influence of temperature on the extraction and total phenolic content yields was also studied using ethanol as the extracting solvent (Figure 3B). The temperature level varied from 50 to 90 °C at a constant irradiation time of 2 min, the ethanol concentration of 20%, the microwave power of 600 W, and the feed-to-solvent ratio of 1:8 for different drying methods. The highest yields of the extract (23.34 ± 0.35% g/g) and total phenolic content (110.25 ± 2.51 mg GAE/g d.w.) were obtained at a temperature of 80°C and the sample was shade-dried. It can be clearly seen that the yields improved when the temperature increased from 50°C to 80°C and then slightly declined when it was beyond 80°C. An elevated temperature could increase the mass transfer of extraction and total phenolic yields by reducing the surface tension and solvent viscosity. In addition, it has been reported that mild heating has the ability to soften the plant matrix, weaken the cell walls, and thus enhance the ejection of bound phenolic compounds (19, 25, 28).
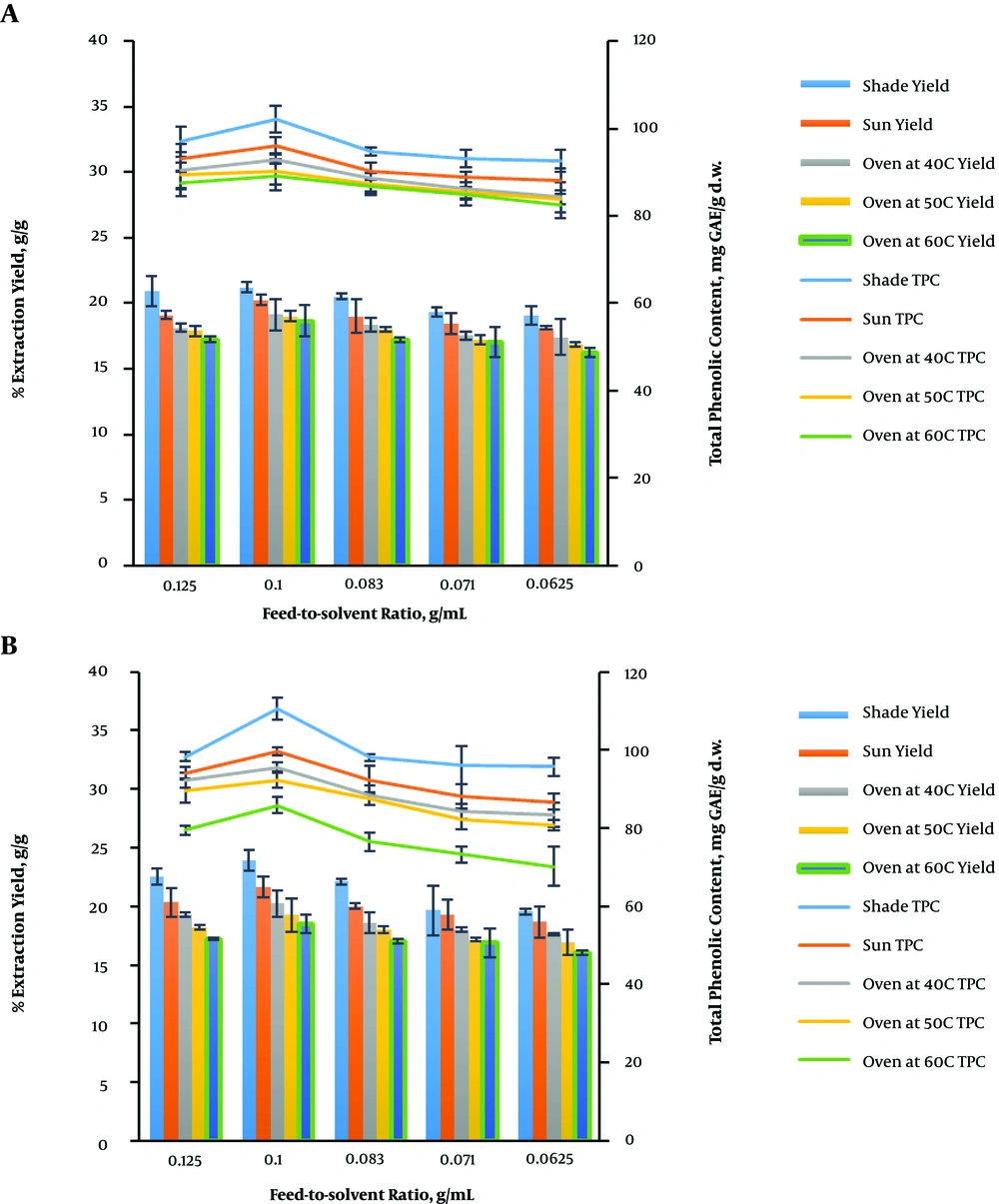
4.4. Effect of Feed-to-Solvent Ratio on the Extraction and Total Phenolic Content Yields of V. amygdalina Leaves
The plant matrix needs to be totally immersed in the solvent for higher recovery during the extraction process. In general, a higher volume of the solvent increases the extraction yields in conventional extraction methods but may result in lower yields in MAE (26, 27, 29, 30). The effect of feed-to-solvent ratio on the extraction and total phenolic content yields of V. amygdalina leaves was studied using water and ethanol as the extracting solvents under different drying conditions (Figure 4). The feed-to-solvent ratio variation (1:8, 1:10, 1:12, 1:14, and 1:16) at a constant irradiation time of 10 min, the microwave power of 400 W, and the temperature of 100°C was investigated when using water as the solvent (Figure 4A). There was a gradual increase in the yields from 1:8 to 1:10 and a further increment in the feed-to-solvent ratio slightly declined the yields. The extraction and total phenolic content yields were in the range of 19.04% - 21.23% g/g and 92.68 - 102.04 mg GAE/g d.w.; 18.14% - 20.24% g/g and 88.05 - 96.14 mg GAE/g d.w.; 17.43% - 19.14% g/g and 84.27 - 92.93 mg GAE/g d.w.; 16.86% - 19.01% g/g and 83.94 - 90.10 mg GAE/g d.w.; and 16.24% - 18.67% g/g and 82.65 - 89.05 mg GAE/g d.w. under shade, sun, oven drying conditions at 40, 50, and 60°C, respectively. The highest values of extraction (21.23 ± 0.36% g/g) and total phenolic content (102.04 ± 3.03 mg GAE/g d.w.) yields were obtained at 1:10 of feed-to-solvent ratio under the shade drying condition.
Figure 4B illustrates the effect of feed-to-solvent ratio (1:8, 1:10, 1:12, 1:14, and 1:16) on the yields at a constant ethanol concentration of 20%, the irradiation time of 2 min, the microwave power of 600 W, and the temperature of 80°C under different drying conditions using ethanol as the extracting solvent. There was a gradual increase in the yields from 1:8 to 1:10 and a further increment in the feed-to-solvent ratio slightly declined the yields. This may be due to the inappropriate stirring of the larger volume of the solvent when microwaves are applied. In addition, a larger volume of solvent may require more absorption of microwave energy, thus resulting in insufficient energy in facilitating the cell wall breakage for effective leaching out of the phenolic compounds (25, 29, 30). The highest extraction (23.88 ± 0.90% g/g) and total phenolic content (110.55 ± 2.92 mg GAE/g d.w.) were obtained at 1:10 of feed-to-solvent ratio after V. amygdalina leaves were shade-dried.
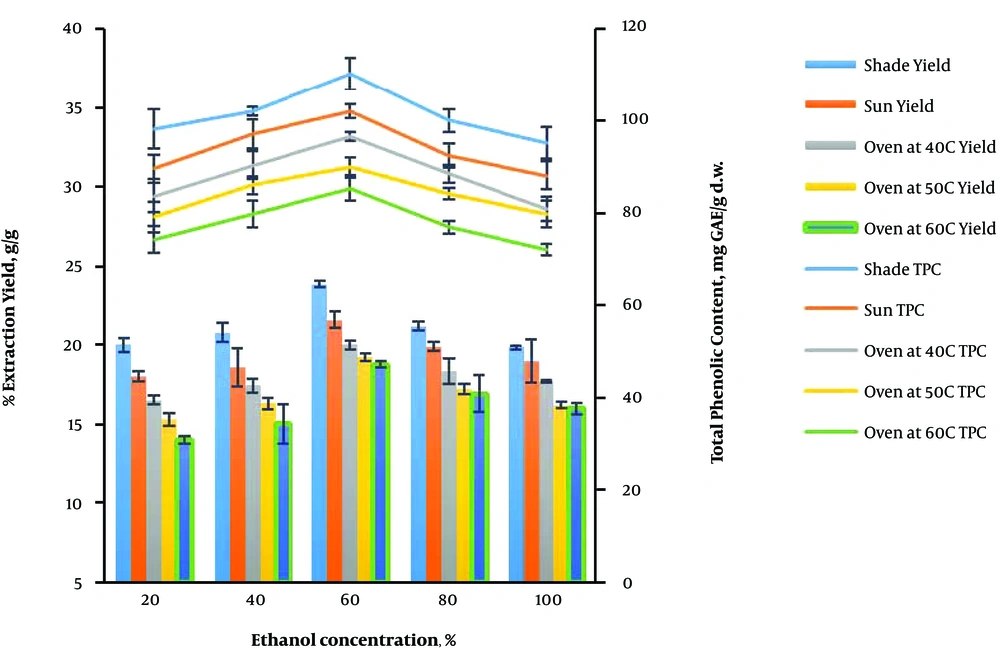
4.5. Effect of Ethanol Concentration on the Extraction and Total Phenolic Content Yields of V. amygdalina Leaves
The effect of ethanol concentration (20, 40, 60, 80, and 100% v/v) on the extraction and total phenolic content yields of V. amygdalina leaves at a constant irradiation time of 2 min, the microwave power of 600 W, and the feed-to-solvent ratio of 1:8 under different drying conditions is shown in Figure 5. The extraction and TPC yields were in the ranges of 19.85% - 23.86% g/g and 95.27 - 110.34 mg GAE/g d.w.; 18.03% - 21.56% g/g and 88.25 - 102.14 mg GAE/g d.w.; 16.54% - 20.00% g/g and 81.05 - 96.62 mg GAE/g d.w.; 15.29% - 19.22% g/g and 79.27 - 90.18 mg GAE/g d.w.; and 14.02% - 18.78% g/g and 72.12 - 85.28 mg GAE/g d.w. for shade, sun, oven drying at 40, 50, and 60°C, respectively.
The yields improved when the ethanol concentration increased from 20% to 60% v/v, and a further increment resulted in the gradual reduction of the yields. The presence of some amount of water can aid the mass transfer process and increase the relative polarity of the solvent, thus enhancing its solubilizing ability through effective swelling of the plant matrix. Therefore, the surface area for solute-solvent interaction increases (24). However, the presence of an excess amount of water can lead to excess thermal stress from rapid heating of the mixture due to effective absorption by water (19, 29). The highest extraction (23.86 ± 0.22% g/g) and total phenolic content (110.34 ± 3.52 mg GAE/g d.w.) yields were obtained when 60% v/v ethanol concentration was used for the extraction of shade-dried V. amygdalina leaves. This showed an improvement compared to the study of Okoduwa et al. that reported the optimum extraction yield of 20.9 ± 1.05% g/g from the microwave-assisted extraction of V. amygdalina leaves using 80% v/v ethanol concentration at 39 min (25).
4.6. The Antioxidant Activities
The IC50 values of the shade-dried sample extracts were determined using DPPH and ABTS radical scavenging assays (Table 1). This is the amount of antioxidant needed to reduce the DPPH or ABTS concentration by 50%, obtained through interpolation from a linear regression analysis whereby a lower IC50 value indicates the presence of higher antioxidant (23). The 60% v/v ethanolic extract exhibited the strongest DPPH and ABTS radical activities of 204 ± 0.92 µg/mL and 31.17 ± 1.4 µg/mL, respectively. Studies have shown that there is a strong positive correlation between antioxidant activities and total phenolic content in the extracts (31). Thus, higher DPPH and ABTS free radical scavenging activities of V. amygdalina leaf ethanolic extract could be due to the presence of a higher amount of total phenolic compounds. This finding was similar to those reported in the literature (19, 32, 33).
| Solvent | % Extraction Yield (g/g) | TPC Yield (mg GAE/g d.w.) | DPPH (µg/mL) | ABTS+• (µg/mL) |
|---|---|---|---|---|
| Water | 21.23 ± 0.36A | 102.04 ± 3.03A | 260 ± 1.10A | 149.78 ± 0.81A |
| 60% v/v Ethanol | 23.86 ± 0.20B | 110.34 ± 3.52B | 204 ± 0.92B | 31.17 ± 1.40B |
a Data are expressed as mean ± standard deviations. Different capital letters along the column indicate a significant difference (P < 0.05).
5. Conclusions
V. amygdalina leaves were screened for the extraction and total phenolic content yields considering different drying methods. Water and ethanol were used as the extracting solvents. The microwave-assisted extraction technique was employed for the one-factor-at-a-time (OFAT) experiments in determining the effect of each extraction parameter. The highest values of yields were obtained from the shade-dried V. amygdalina leaves. In addition, the solvent used in the extraction had an effect on the recovery yields of the extracts. The obtained recovery yields were higher from ethanolic extracts than from aqueous extracts. Therefore, a further study is in progress on the optimization of the extraction parameters using the obtained range of values.