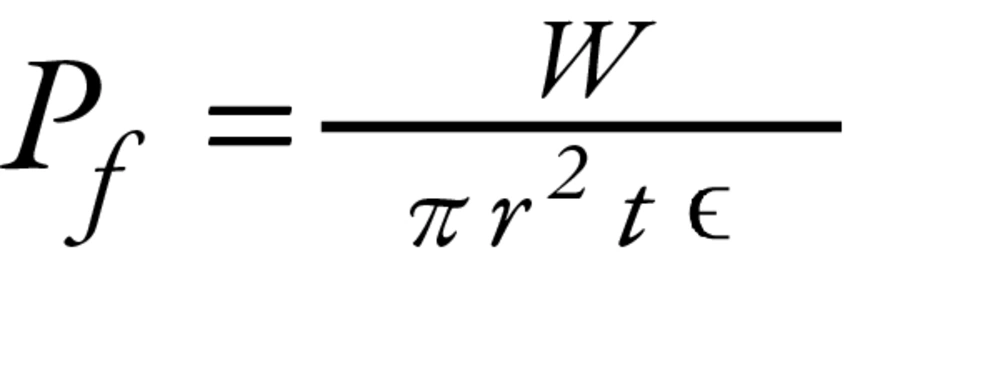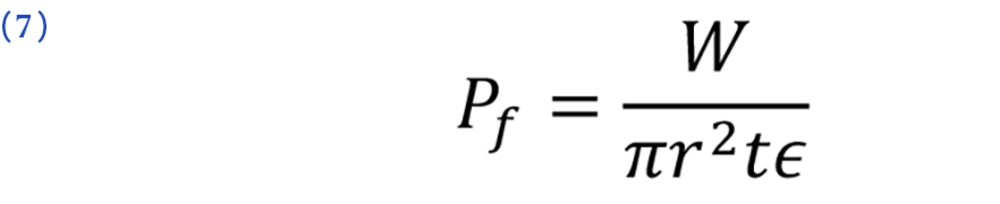1. Background
ϵ The main purpose of this study was to compare the effect of warm and cold drying, in this case oven and freeze-drying, on the mechanical properties of quince seed mucilage in different granule sizes. The most common mechanical properties for a tablet during production processing are yield stress, elongation, hardness, and toughness. The first two are measured by a tensile test, where a sample is loaded until it begins to undergo plastic strain (i.e. strain that is not recovered when the sample is unloaded); the stress is called the yield stress (1). Insight into mechanical properties are critical to understanding powder processing for tableting, and the various parameters that reflect these basic fundamental properties of powder are very important in tableting, especially when a solid dosage form involves processing multi-particulate powder which is heterogeneous in shape, size, and size distribution. Tablet bond strength and capping/lamination tendency, as measured by tensile strength (T) and the brittle fracture index (BFI), are important parameters that determine the mechanical properties of compressed compacts (2, 3). Therefore, when it is not possible to compact a powder (especially a gum) directly, the attractive way to cure the powder is to go through a dry or wet granulation, preferably a wet one.
It is not certain whether an increase or a decrease in particle size will influence tablet mechanical characteristics. Therefore the particle size and its distribution are important, though often ignored, parameters of a powder bed that should be considered during compaction into tablets (4-6). Another important factor which could influence the texture, density, porosity, and the other mechanical, physical, and chemical properties is the type and condition of the drying process (7, 8). Depending on the drying process, the same raw material may end up as a completely different product. The most commonly used drying techniques for various plant gums include oven, spray, freeze, and vacuum drying (9).
In the past, there have been important developments on dried plant polymers, such as gums and mucilage, due to their diverse pharmaceutical applications and use as adjuncts or excipients (10). But, in recent years researchers have been focused on the use of biopolymers as semi-drugs that take part in the function of a drug, such as prolonging or enhancing intranasal drug delivery (11, 12), or as real drugs with independent active action (13, 14). Some studies have examined the animal dose response to quince seeds mucilage, either on airway disorders (13) as a relaxant or because of its wound-healing properties (14). These findings encourage us to make a new and therapeutic tablet dosage form of this biopolymer. The seeds of Cydonia vulgaris pers (Cydonia oblonga mill) belonging to the Rosacea family are found in central Asia, and commonly known as quince seeds in English, Beedanna in Gujarati, and Behdane in Persian. They contain a high proportion of mucilage medicinally used as a demulcent in treatment of asthma, bronchitis, dysentery, diarrhea, sore throat, and cough. Other uses of the plant are as a source of flavor in marmalade, liqueur, candies, and jelly (13); a tablet binder; (15) and a super-disintegrating agent (16). Tablets are the most common solid dosage forms, and are administrated orally, but many patients, especially children, mentally ill patients, and geriatrics, have problems swallowing the tablets (17). Furthermore, the action of some drugs should be localized and have an extended effect exactly on the site of conflict, such as those treating airway disorders.
2. Objectives
Generally, the first step for preparing a tablet is to study the flow properties of a powder, and also the capability of its granule to have an acceptable compressibility in different sizes or conditions of preparation. Therefore, the aim of this research was to develop a mouth-dissolving troche compressed tablet that disintegrates gradually to spread on the throat, epiglottis, and airway cells, for as long as the drug is available.
3. Materials and Methods
Quince seeds from Isfahan were purchased from a local market, and then identified as Pyrus oblong by the botanical department of the University. All the other chemicals used, such as magnesium stearate and regents, were of analytical grade. Instruments used were a freeze drier (Vd-60-Japan), hot air oven (AS, Iran), weighing balance (PT 120, Sartorius, Germany), American sieves with different meshes, sieve shaker (Erweka, Germany), Karl Fisher auto titrator (M/S, Metrohm), Monsanto hardness tester (Pfizer type), single-punch tableting machine (Erweka, K0, Germany), and Erweka Friabilator (TAR200, Germany). The other materials and instruments used in this research were the same as those published in the first part of this study (18).
3.1. Preparation of QSMB Granules
A quantity of 300 g of each QSMB powder previously dried by oven- (warm) and freeze-drying (cold) methods (18) was granulated by deionized water. The wet mass was then passed through an American sieve No. 8 and dried at temperature not exceeding 50°C in a hot air oven for 1 hour, retaining about 3% moisture content.
3.2. Determination of QSMB Moisture Content
The moisture percent was calculated using a Karl Fischer auto titrator (M/S. Metrohm). A definite amount of QSMB was dispersed in methanol, stirred to extract water, and then titrated with a standardized Karl Fischer reagent until the end point was reached. Moisture content was calculated using the following Equation:
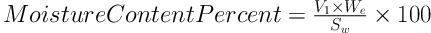
Where V1 is the volume of the Karl Fischer reagent, We is the water equivalent, and Sw is the sample weight in milligrams. Fifteen mL of the reagent was equivalent to 75 mg of water, and the water equivalent was 5.2.
3.3. Mechanical Evaluation of the Granules
3.3.1. Particle Size Distribution
The dried QSMB granules were classified into five different sizes (212, 500, 710, 850, and 1700 microns) by shaking them for 10 minutes with a nest of the aforementioned sieve mounted on a sieve shaker (Erweka). The weight of each size was obtained afterwards.
3.3.2. Micromeritic Properties of the Granules
Prior to compression of the tablets, the granules were evaluated for the following characteristic parameters to ensure their suitability before compression.
3.3.3. Angle of Repose
This parameter (θ) was determined by the fixed funnel method (19). A funnel was clamped with its tip 2 cm above graph paper placed on a flat horizontal surface (h). The QSMB was carefully poured through the funnel until the apex of the cone thus formed just reached the tip of the funnel. The mean diameters (D) of the base of the powder cones were determined and the tangent of the angle of repose was calculated using the following Equation:
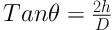
3.3.4. Bulk and Tapped Densities
In a triplicate determination process, a 2.0 g quantity each of the granule samples (W) was placed into a 10 mL measuring cylinder without any tapping, and the volume (V0) occupied by each one was noted. The occupied volume after 100 taps of the table (V100) was read again. Finally, the bulk and tap densities were calculated as the ratio of weight to volume, as follows (20):
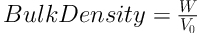
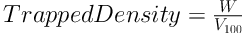
3.3.5. Hausner Ratio and Carr’s Index (Compressibility%)
Based on the bulk (B) and tap (T) densities, both the Hausner ratio (HR) and the compressibility percent (C%) was calculated with respect to the following Equations (21, 22).
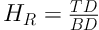
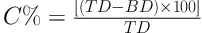
3.4. Preparation of the Tablets
A single-punch tableting machine (Erweka, Ko, Germany) was used for the preparation of the tablets from the various sizes. Magnesium stearate (1%) was used to lubricate the granules and machine compression devices. 500 samples, each weighing 800 ± 5 mg, were compressed into tablets of 20 ± 0.4 mm diameter and 5 ± 0.5 mm thickness, at a fixed arbitrary pressure load of 8 on the load scale. The tablets were kept overnight in a fixed condition such as a desiccator.
3.5. Tablet Packing Fraction Evaluation
This value (Pf) was calculated from the particle densities of the tablets prepared from each size fraction and determined by the liquid paraffin displacement method, using the following Equation (2, 23):
where W is the mean weight, r is the radius, and t is the thickness of the tablets. The particle density is shown by ϵ and was previously tested by the liquid paraffin displacement evaluation.
3.6. Tablet Tensile Strength Measurement
Ten tablets were randomly selected from each size classification for testing the tensile strength, using a Monsanto harness tester, Pfizer type (2, 24). To calculate the tensile strength (T), the load (P) required to cause a diameter fracture in a tablet is needed. This parameter can simply be determined by a hardness tester. The mean fraction load of triplicate determinations was applied to calculate the tensile strength of tablets with particle sizes, using the following expression, where t and D are the thickness and diameter of the tablet, respectively.
3.7. Friability of the Tablets
This parameter was determined by using an Erweka friabilator (TAR2OO, Germany) on the ten selected tablets from each different particle size. The tablets were initially weighed and installed in the friabilator. The tablets were rotated for 5 minutes at 20 rpm and weighed again after dusting from the separated particles. The tests were carried out in triplicate for the various particle fractions, and the friability percents were calculated.
3.8. Hardness Evaluation of the Tablets
The hardness of the tablets was determined by using a Monsanto hardness tester (Pfizer type) and expressed in kg/cm2.
3.9. Statistical Analysis
The data were presented as mean ± standard error of the mean (SEM). The significance level was calculated by one way analysis of variance (ANOVA) using the post-hoc test. P values of 0.05 were considered significant.
4. Results
4.1. Moisture Content
The moisture content of the QSMB granules for the oven- and freeze-dried mucilage powder was found to be 3% ± 0.1% and 3.5% ± 0.1%, respectively.
4.2. Mechanical and Physical Properties
The mechanical parameters results for the freeze- and oven-dried QSMB are shown in Tables 1 and 2, respectively.
| Parameter | Particle Size, µm | ||||
|---|---|---|---|---|---|
| 212 | 500 | 710 | 850 | 1700 | |
| Bulk density, g/cc | 2.5 (0.1)b | 2 (0.08) | 1.5 (0.08) | 0.8 (0.05) | 0.5 (0.02) |
| Tapped density, g/cc | 4.5 (0.2) | 3.5 (0.15) | 3 (0.1) | 1.5 (0.08) | 0.65 (0.03) |
| Compressibility index, % | 35 (1) | 30 (0.8) | 24 (0.7) | 19 (0.5) | 15 (0.25) |
| Hausner ratio | 1.5 (0.08) | 1.4 (0.07) | 1.3 (0.06) | 1.25 (0.05) | 1.1 (0.01) |
| Porosity, % | 8 (0.9) | 11.5 (1) | 13 (1) | 15.5 (1) | 17 (1.1) |
| Repose angle, ° | 49 (2) | 45 (1.8) | 40 (1.7) | 35 (1.6) | 25 (1.5) |
Mechanical Properties of Different Size Fractions of Quince Seed Granules, Dried by the Freeze-Drying Methoda
| Parameter | Particle size, µm | ||||
|---|---|---|---|---|---|
| 212 | 500 | 710 | 850 | 1700 | |
| Bulk density, g/cc | 3.5 (0.14) | 2.4 (0.13) | 1.9 (0.1) | 1.1 (0.08) | 0.8 (0.02) |
| Tapped density, g/cc | 5.5 (0.15) | 4 (0.11) | 3 (0.1) | 1.9 (0.08) | 0.85 (0.05) |
| Compressibility index, % | 45 (1.1) | 39 (1.05) | 32 (1) | 28 (0.8) | 25 (0.7) |
| Hausner ratio | 2 (0.08) | 1.8 (0.07) | 1.6 (0.06) | 1.3 (0.05) | 1.5 (0.06) |
| Porosity, % | 6 (0.8) | 9 (0.9) | 11.5 (1) | 13 (1.05) | 15 (1.1) |
| Repose angle, ° | 60 (2.1) | 54 (2) | 45 (1.9) | 38 (1.8) | 31 (1.6) |
Mechanical Properties of Different Size Fractions of Quince Seed Granules, Dried by the Oven-Drying Methoda
4.3. Hardness (Breaking Force) and Friability of QSMB Tablets
The results of the effects of drying method and granule size on the breaking force (harness) and the friability are presented in Table 3.
| Particle Size, µm | Hardness (Breaking Force), Kg | Friability, % | ||
|---|---|---|---|---|
| Freeze-Dried | Oven-Dried | Freeze-Dried | Oven-Dried | |
| 212 | 2.9 (0.1) | 2 (0.1) | 1.1 (0.2) | 1.5 (0.3) |
| 500 | 3.5 (0.2) | 2.5 (0.1) | 1 (0.1) | 1.3 (0.2) |
| 710 | 3.7 (0.2) | 2.8 (0.2) | 0.9 (0.1) | 1.1 (0.1) |
| 850 | 3.9 (0.2) | 3 (0.2) | 0.75 (0.15) | 0.9 (0.1) |
| 1700 | 5 (0.2) | 3.5 (0.1) | 0.7 (0.1) | 0.85 (0.15) |
Comparison of Hardness (breaking force) and Friability Results of Different Size Quince Seed Tablet Dried by Oven- and Freeze-Dryinga
4.4. Packaging Fraction and Tensile Strength
The result of the effects of drying method and QSMB granule size fractions on the packing fraction (Pt) and tensile strength of the tablets are shown in Table 4.
| Particle Size, µm | Packing Fraction | Tensile Strength | ||
|---|---|---|---|---|
| Freeze-Dried | Oven-Dried | Freeze-Dried | Oven-Dried | |
| 212 | 0.87 (0.2) | 0.75 (0.2) | 1.7 (0.3) | 1.65 (0.15) |
| 500 | 0.89 (0.2) | 0.77 (0.1) | 1.6 (0.2) | 1.55 (0.1) |
| 710 | 0.9 (0.1) | 0.79 (0.2) | 1.55 (0.1) | 1.45 (0.2) |
| 850 | 0.92 (0.1) | 0.8 (0.3) | 1.52 (0.2) | 1.31 (0.2) |
| 1700 | 0.96 (0.2) | 0.82 (0.3) | 1.45 (0.2) | 1.3 (0.1) |
Comparison of Packing Fraction and Tensile Strength of Quince Seed Granules, Dried by Oven- and Freeze-Dryinga
5. Discussion
The moisture content of a powder which is provided for a tableting program can influence the flow, compression, and stability properties of the granule powder. Also, sometimes the moisture may become a critical factor if its amount passes ranges of 3 to 4 percent, depending on the texture and crystallization properties of the powder (25). Therefore, the moisture result of 3 and 3.5 percent for oven and freeze-dried granules, respectively, could be optimum amounts because the flow and compression did not show any difficulty during and after tablet processing. Furthermore, the granules (powders), especially in mucilage (or gum) with an adhesive nature, might show weak flowability when they have uncontrolled moisture. It is important to investigate the moisture content of materials, particularly in case of industrial production. This factor may show an influence from a critical to optimum range of activity on the overall process of tableting (26, 27). The other physical properties such as bulk and granule densities, inter-space porosity, particle size, and size distribution are likewise critical parameters for controlling the quality and functional properties of the powders (granules). The bulk and tapped densities give us a picture and description of the packing and arrangement (or rearrangement in the case of granulation) of the particles, and the compaction specifications of the material (28).
The drying processing also significantly affects the bulk density, which depends on the attractive inter-particle forces, particle size, and number of contact positions (7). Comparing the two methods of drying the QSMB primary mucilage powder in terms of the resulting mechanical and physical properties, particularly density, compression characteristics, porosity, and angle of repose, showed some significant differences (18). This difference is very significant (P < 0.05) and important specially for the compressibility index, or even for the other parameters, because it defines which powder could have better compaction and compressibility properties during the tableting process. Since the compressibility index and Hausner ratio are 15 to 35% and 1.1 to 1.0 for different size fractions of the freeze-dried powder, compared to 25 to 45% and 1.5 to 2 with the oven-dried powder, the freeze-dried powder is in better mechanical condition to form a qualified tablet.
It seems that there is a direct relationship between mechanical movement properties (e.g. flowability) for the particle size range of 212 to 1700 m for QSMB. This concept of different particle size fractions has also been reported by Eichie and Kudehinbu in their research on a chemical paracetamol tablet (2). An important question about the different particle size fractions is which fraction would be the most optimum for to prepare a QSMB tablet with 800 mg weight. The most probable answer is the fractions with the range 850 - 1700 m, which created a density two times that of the tablets formed from 212 m. Other advantages could include a desirable mechanical movement and an external elegancy, due to the optimum and conventional relationship between the weight, surface area, and particle size distribution of a tablet. As shown in Table 1, an increase in granule size causes a decrease in the granule density, and therefore an increase in the porosity % of the tablets. This inverse relation between the granule size and density, and also the direct relationship for porosity %, have been reported by other studies (2, 29, 30), and may be significant (P < 0.05) but not for density.
Eichie and Kudehinbu (2) reported that the reason for high porosity of the bigger granules (850 - 1700 m) was probably the presence of a larger void space, a feature characteristic of large particles, and a limited surface area available for inter-particulate bonding. Of course, the type of drying (e.g. lyophilization) could have its own influence, too. Statistical evaluation of the results in Table 3 showed that the freeze-dried method has a significantly better difference (P < 0.05) for different size fractions, at least in some cases. It should be notified that the significant differences between the smallest (212) and biggest fractions are thoroughly obvious (P < 0.05). Another important point is the probability of a direct relationship between the friability of the tablets and the decreased particle size fractions, which is very important in transportation problems. The final interpretation of friability for all different size fractions and drying methods (Table 3) is that all tablets had a friability index of less than 1%, except those made from fractions 212 and 500 m for the freeze-drying method and 710+ µm for the oven methods, which is in conformity with the official standard of pharmaceutical reference books (USP).
In Table 4, an increase in the particle size resulted in an equivalent increase in the packing fraction (Pt) of the prepared granules and tablets. The difference is not significant (P > 0.05) from each size to the next, but is significant (P < 0.05) between the least and greatest particle size fractions. This trend was also reported by Eichie and Kudehinbu for acetaminophen as a chemical drug (2). It should be notified that a high packing fraction value for the particles (granules) of QSMB could be an indication of an optimum degree of consolidation to compact and establish a qualified tablet. Based on the reports of researchers who worked on the similar subjects, this situation might depend on some successive events that follow the compression processes, like repacking, deformation, fragmentation, and bonding formation (2, 31). Furthermore, it seems that there is an intensive inclination to larger particles (granules) for deformity and fragmentation, forming a larger number of bonding points at the time of compression compared to the smaller granules. This occurrence, and also the function of granulation, encourages the plasticity of the particles. It might be increased for the larger granules, too. Therefore, the plasticity of the large granules may have an increase in the surface of the fragments, creating greater particle to particle contact and also better bonding for closer packing (30). Of course, this phenomenon or mechanism occurs in significantly different ways (P < 0.05) in the powder that was freeze-dried compared to the oven-dried result, which is obvious from the results shown in Table 4. As was stated in the first part of this research (18), oven drying made the yielded powder brown, which when compressed into a tablet became apparent as dark and dotted faces on both sides of the tablet (Figure 1).
This is not good for the face (surface) of a tablet, but when the freeze-dried powder was compressed, a consistent white texture was obtained on both faces of the tablet (Figure 2).
Of course, the whiteness and especially the consistent, dot-free texture is an advantage for a tablet. Therefore, it should be noted that freeze-drying is one way to purify a drug and to decolorize it as much as possible. Compared to chemical and harmful bleaching (32), which could be very dangerous for oral material, freeze-drying for purification is a safer method.