1. Background
Foot and mouth disease or FMD is a prevalent and contagious disease of several animals, including wildlife and livestock with cloven hooves (1). The disease’s clinical symptoms in vulnerable species are fever; vesicular lesions in the mouth, feet, muzzle, and teats; and lameness (2). The etiologic agent, the FMD virus (FMDV), belongs to the Aphtovirus genus from the Picornaviridea family. FMDV exists as a disease agent in seven particular serotypes: Asia 1, SAT 1, SAT 2, SAT 3, O, A, and C. Vaccination or infection against one serotype does not cross-protect against other serotypes; therefore, constant surveillance and testing potential cures is essential (3). Recently, FMD outbreaks in developed and developing countries, and their related adverse economic impacts, have expanded universal concern among governments (4). In Iran, FMD is the most serious cattle disease and the most prominent minatory factor for dairy industrials. According to cattle-disease defeating programs, it’s considered the first disease to be overcome.
Alhagi maurorum Medik belongs to the Fabacea family and Fabales order. Fabacea comprises approximately 730 genera and 19,400 species. Plants belonging to this family are known to produce a large number of biologically important secondary metabolites. A. maurorum is one of the species of legumes, and it’s also known as camel thorn, Caspian manna, or Persian manna-plant. The camel thorn bush is native to the region ranging from the Mediterranean to Russia, and is very abundant in the Middle East. It is a perennial shrub which has an extensive rhizome system that may reach a depth of six feet in the ground. It has small, pink to red pea flowers, and brown legume pods (5). Various studies have shown that A. maurorum has been used in folk medicines. It is used in diuretic, diaphoretic, and anti-ulcer treatments, and also has tissue-repairing properties (4, 6). The plant is used in the treatment of diseases of the liver and urinary tract, and also as a laxative (7). Oil extracted from the leaves is applicable for the treatment of rheumatism, and the flowers are used for warts and migraines (8). A study by Sulaiman showed that this plant has anti-bacterial properties (9).
2. Objectives
Considering the healing and anti-bacterial properties of the extract, and also that traditional stock breeders use it to treat FMD lesions, the antiviral effects of the hydro alcoholic extract of A. maurorum were investigated.
3. Materials and Methods
3.1. Preparation of Extract
The plant samples were collected from Shahid Chamran University’s farmland in Khuzestan province, Iran, in April of 2014. The taxonomic identification of these plants was done by comparing them with the existing herbarium in the biology department of Shahid Chamran University. The aerial parts of the plants were taken out, cleaned, and dried in the shade for 2 weeks, and then powdered. Extraction was done via maceration. 24 gr of powdered aerial parts were extracted with 100 mL of 80% methanol. The solution was filtered and kept at room temperature to evaporate the solvent. The pH of the extract was also determined, to judge if it would damage either viruses or cells.
3.2. Cell Culture and Virus Culture
A bovine kidney cell line which is known as Razi bovine kidney (RBK) cells was obtained from the Pasteur institute, Tehran, Iran. The RBK cells were cultured in a growth medium containing RPMI1640 (Bioidea, Iran) and supplemented with 10% fetal bovine serum (FBS, University of Tehran product, Iran), 100 units/mL penicillin G, and 100 μg/mL of streptomycin (penesterp) (Bioidea, Iran), and were kept at 37°C in an incubator with 5% CO2. The FMD virus type O IRN/1/2010 was provided by the faculty of veterinary medicine, University of Tehran. 300 µL of the virus was inoculated into the flask covered with a RBK monolayer and incubated for 1.5 hours, then the virus was removed and the cells were washed with phosphate buffer saline (PBS). The culture medium with 2% FBS was added and incubated for 3 days until cytopathic effects (CPEs) appeared. The supernatant of the cell culture was removed as a source of the virus.
3.3. Cytotoxicity Assay
The cytotoxic potential of the extract was determined by an MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) assay (10). The MTT colorimetric assay was performed in 96-well plates. Cells were seeded at a concentration of (5 × 105) cells/well and incubated for 48 hours at 37°C in 5% CO2. Microscopic observation ensured that normal cell layers were preserved in each well throughout each experiment. After treatment with various concentrations of the test compound (300, 30, 3, 0.3, 0.03, and 0.003 mg/mL with 3 wells for each concentration) the cells were incubated for an additional 72 hours at 37°C. After that, the medium was removed and the cells in each well were incubated with 10 μL of the MTT solution (5 mg/mL) (Sigma-Aldrich Chemical, Germany) for 4 h at 37°C. The MTT solution was then discarded and 150 μL isopropanol was added to dissolve the insoluble formazan crystals. The plates were incubated at 37°C for 15 minutes. The optical density (OD) was measured at 580 nm using an ELISA reader (BioRad, USA). Percentage of inhibition (PI) was determined by the following Formula 1:

Where At is the absorbance of the test sample and As is the absorbance of the control sample without the extract. The CC50 (50% cytotoxic concentration) was calculated from dose-response curves (10).
3.4. TCID50 Titration Assay
500 µL of a cell suspension containing 5 × 105 cells was added to 24-well plates, and after formation of a cell monolayer, the media was removed and the wells were washed with PBS. Ten-fold serial dilution of the virus (1 - 10-7) was prepared, and 50 µl of each dilution was added to 24-well plates (3 wells for each concentration). The plates were incubated at 37°C for 1.5 hours, then washed with PBS and supplemented with 2% media. Afterward, the plates were incubated for 72 hours, until CPEs appeared. A calculation of TCID50 was done using the Reed & Muench method (11).
3.5. Antiviral Activity
The antiviral activity of the tested compounds was evaluated by measuring the reduction in CPEs and calculating and comparing the TCID50 values, before and after treatment with the extract. Briefly, monolayers of RBK cells were prepared in 24-well plates. 250 µL of a non-cytotoxic concentration of the extract (3 µg/mL) was added to 250 µL of ten-fold serial dilutions of the virus ranging from 1 to 10-7, and kept at 4°C for 1.5 hours. Then, 100 µL of each dilution (virus/extract) was added to each well of the plate (3 wells for each concentration) and incubated at 37°C. After 1.5 hours they were washed away with PBS, and 500 µL of the culture medium with 2% FBS was added. The plates were kept in an incubator for 72 hours until CPEs appeared.
3.6. Statistical Analysis
Each experiment was repeated a minimum of 3 times, and data are presented as mean ± standard deviation. For the analysis of data, we used the SAS program and a Duncan test. P values equal to or less than 0.05 were considered significant.
4. Results
4.1. Cytotoxicity
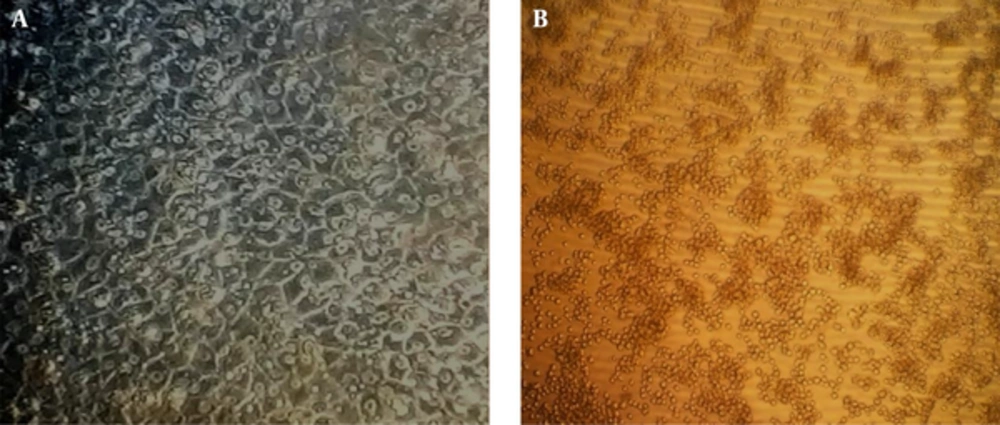
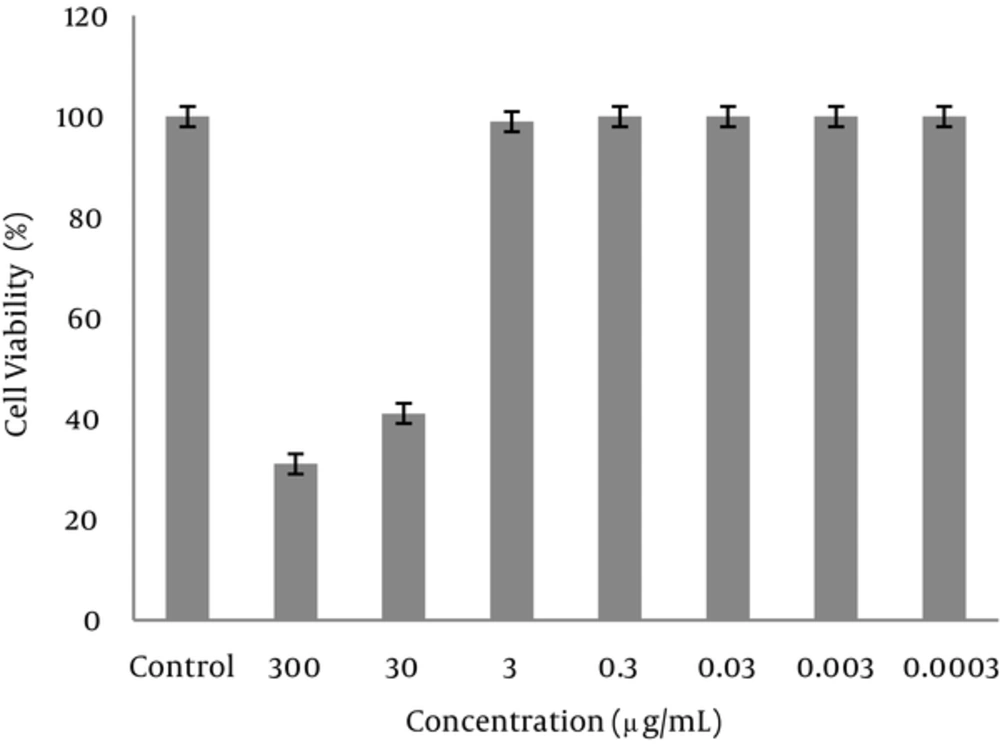
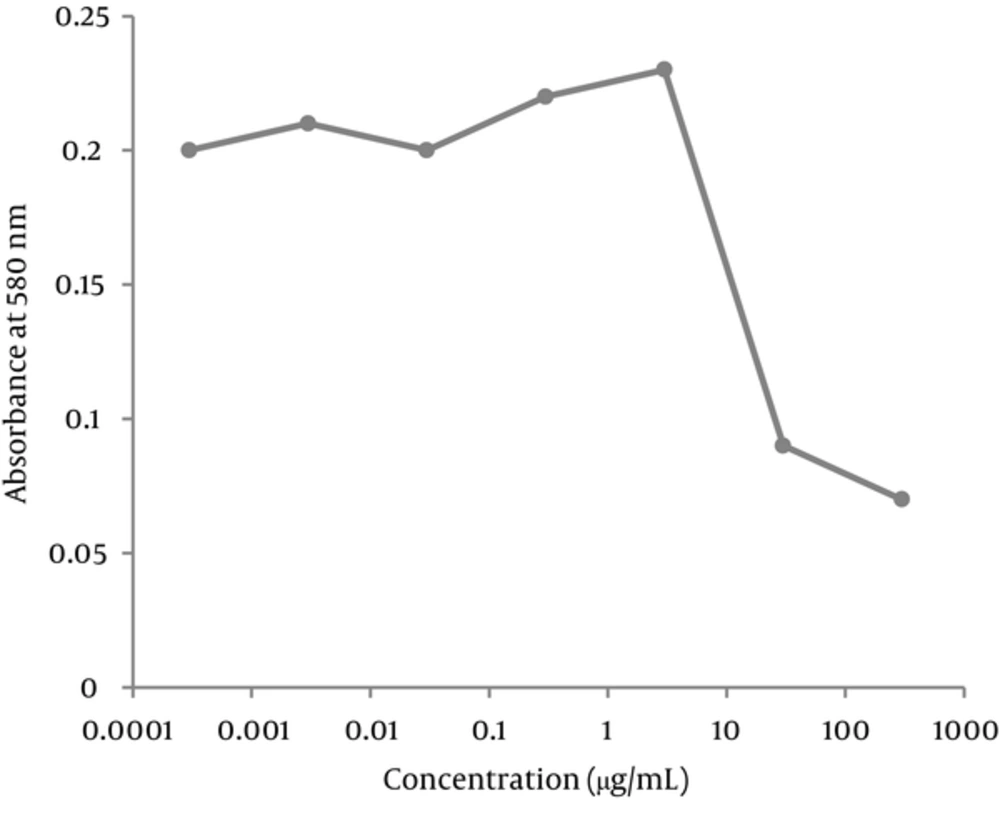
The morphology of the cells in the presence of 300 μg/mL of extract was deformed after 24 hours. The cells treated with 30 mg/mL of the extract were partially deformed after about 48 hours, but the presence of 3 μg/mL or less had no effects on the cells. Cytotoxicity of the extract was measured by an MTT assay. Based on the results of this assay, the CC50 was about 30 μg/mL and concentrations equal or below 3 μg/mL had no cytotoxic effect (Figures 1-3).
The impact of A. maurorum on cell morphology was visualized by an inverted microscope (Olympus) (40x magnification). a, untreated RBK cell line (cell control); b, treatment of RBK cells with 300 μg/mL A. maurorum for 72 hours. Cell death and changes in cell morphology were observed at this concentration.
4.2. Virus Culture and TCID50
After the formation of a cell monolayer, serial dilutions of virus were inoculated and CPEs appeared after 72 hours. The titer of the virus was calculated based on the Reed & Muench method (11). According to this, the titer of the virus was gain 2 × 103.5 TCID50 /mL.
4.3. Measurement of Antiviral Activity of Extract
The TCID50 of virus without presence of the extract was 2 × 103.5/mL. Otherwise, a non-toxic concentration of methanolic extract of camel thorn adjacent to FMDV produced no observed CPEs; therefore, the TCID50 was calculated as zero. Hence, methanolic extract could reduce the infectivity of the virus by about 2 × 3.5 log (6324.56 times). The pH of the extract was nearly neutral (6.7); therefore, it can be concluded that it wouldn’t kill the viruses.
5. Discussion
Foot and mouth disease is one of the most prevalent cattle diseases that can damage the husbandry industries, but there is no precise treatment; hence, investigating novel methods for treatment is valuable. Plants are significant sources of possibly useful structures for the development of novel chemotherapeutics (7). Some studies have investigated the antiviral effects of different compounds on the FMD virus. Qian et al. (12) found that flavonoids, especially epigenin, can inhibit the replication and cytopathogenic effects of FMDV by suppressing internal ribosome entry site-driven translational activity. Another study showed that meilacine, a peptide isolated from Melia azedarach L, inhibits the multiplication of different strains of FMDV in BHK-21 cells. This prevents the uncoating step and blocks virus penetration. Meliacine also inhibits the low-pH-induced fusion of infected cells and interferes with the release of new particles (13). A study by Choi et al. (14) similarly showed that raoulic acid from Raoulia australis has antiviral activity against picornaviruses.
The current study addresses the anti-FMDV and cytotoxic effects of the local plant A. maurorum Medik, harvested from Ahvaz, Iran. The selected plant was based on its traditional uses as a folk medicinal plant for the treatment of various diseases (7), and because various studies have proven this plant’s medicinal properties as an anti-oxidant, anti-inflammatory (8), anti-ulcerogenic (15), and anti-diarrheal agent (7). One study showed that methanolic extract of A. maurorum could induce an inhibitory effect against the proliferation of HL-60 cells. In this study the IC50 values of the leaves and flowers extract were 16.0 and 22.0 μg/ mL, respectively (9). An earlier study by Behzad et al. (16) showed A. maurorum methanolic extract has no cytotoxic effect against human lung, colon, liver, or breast cancer cell lines, or against one normal bovine cell line. The cytotoxicity analysis in our study is in accordance with previous published studies.
Abd-Ellatif et al. showed the antimicrobial activity of ethanolic extract on Fusarium oxysporum, Alternaria alternate, Aspergillus flavus, Bipolaris oryzae, Fusarium solani, Chetomium sp., and Mucor sp. (17). Another study showed methanolic extract antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhimurium, and Candida albicans (9). Zain et al. (18) showed that the ethanolic extract of A. maurorum has antimicrobial activity against various bacteria, as well as unicellular and filamentous fungi.
Our study showed that camel thorn has antiviral activity, which is probably the cause of its use in traditional medicine. The antiviral effects of camel thorn can be justified on the grounds of chemical compounds such as flavonoids which give the plant its medicinal importance. Phytochemical analysis of the A. maurorum extract showed the presence of flavonoids, alkaloids, glycosides, saponins, tannins, steroids, and anthraquinone as major constituents (19-21). Several additional constituents were reported in the extract: β-sitosterol, coumaric acid, and hydroxybenzoic acid (22). That antiviral activity of the extract might contribute directly to flavonoid or coumarin compounds (6). Therefore, the chemistry of plant natural products and their biological effects have been the focus of intensive research, and the effects are explained in terms of chemical constituents. However, the precise mechanism of the antiviral activity of chemical compounds in these extracts remains to be clearly determined. Further studies should explore the molecular mechanisms of the A. maurorum extracts, and determine its properties in vitro. The present investigation supports the use of the methanol extract of A. maurorum as a disinfectant in folk and modern medicine, and the antiviral activity may justify its use as a source of natural antiviral compound to protect animals from foot and mouth disease.