1. Context
Cisplatin (cis-diamminedichloroplatinum [II]; CDDP; Platinol) is a platinum-based antineoplastic agent that serves a highly effective treatment regimen for an array of malignancies such as head and neck cancer, cervical cancer, soft-tissue neoplasms, squamous cell cancer, non-small cell lung cancer, gastric cancer, testicular cancer, bladder cancer, and ovarian cancer (1, 2). Despite its effectiveness, the clinical use of cisplatin is compromised in up to 85% of cases due to severe side effects including ototoxicity, nephrotoxicity, bone marrow toxicity, gastrointestinal toxicity, and peripheral neuropathy (3). The prevalence of cisplatin nephrotoxicity was reported as 34.1% among various cancer patients in a study (4) and the incidence of cisplatin-induced nephrotoxicity was around 30% - 40% in another study (5), which was dose-dependent and usually reversible. It has been reported that CDDP, as a platinum-based alkylating compound, has an ability to interact with DNA to form interstrand cross-links and intrastrand bifunctional N-7 DNA adducts at d(GpG) and d(ApG) (6). The formation of these adducts can result in DNA damage, oxidative stress, protein synthesis inhibition, and mitochondrial dysfunction (7).
The current treatment strategy for cisplatin-induced nephrotoxicity (CIN) manifestations primarily includes supportive care with sodium chloride or bicarbonate volume expansion, metformin withdrawal, administration of various agents such as nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, angiotensin II-receptor blockers and statins, and reduced volume of contrast media (8). CIN can be ameliorated using oral hydration therapy (9) or magnesium supplementation (7); however, it is reversible and does not completely prevent CIN. Dialysis has been successfully implicated in the management of various features of CIN including uremia, metabolic disturbances, and hypervolemia; however, it does not play any role in the removal of accumulated cisplatin from renal tissue (10). Despite these various strategies, the treatment of CIN still poses limitations.
The accumulation of toxins causes the elevation of reactive oxygen species (ROS), which, in turn, leads to a variety of consequences (11, 12). The decreased levels of antioxidant enzymes including superoxide dismutase (SOD), glutathione reductase (GSH-R), and catalase (CAT) can lead to the structural alteration in the cell membrane, which, in turn, causes apoptosis and cell death (13, 14). Some compounds with antioxidant potential such as vitamin E, selenium, glutathione, N-acetyl-L-cysteine, and lipoic acid have an ability to bind with ROS and inhibit the damage produced by ROS (15, 16). Numerous randomized controlled trials have been carried out over the past few decades and have shown the potential of these antioxidant moieties against CIN in various cancer patients (17-22). However, conflicting evidence makes us unable to provide any robust results for the use of antioxidants against CIN.
2. Objectives
To the best of our knowledge, no meta-analysis has been carried out to determine the efficacy of antioxidants against CIN. Hence, the aim of the present study was to investigate the efficacy of antioxidant supplements on CIN through a comprehensive meta-analysis of randomized controlled trials.
3. Methods
3.1. Data Sources and Selection Criteria
This systematic review and meta-analysis study was conducted as per the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (23) (Appendix 1 in Supplementary File).
3.2. Quality Assessment of the Articles
The quality of each study included in the analysis was assessed using the Cochrane risk of bias tool for systematic reviews of interventions (version 5.0.1) (24) and the Downs and Black critical appraisal tool. Two independent reviewers performed the quality assessment and disagreements on scores were resolved through discussion.
3.3. Data Analysis
The standardized mean difference (SMD) was used with a 95% confidence interval (CI) and standard deviation (SD). A meta-analysis was conducted with the simultaneous use of random-effect models. All statistical analyses were performed using RevMan V.5.3 software (Cochrane Collaboration, Oxford, UK).
4. Results
4.1. Summary of Included Studies
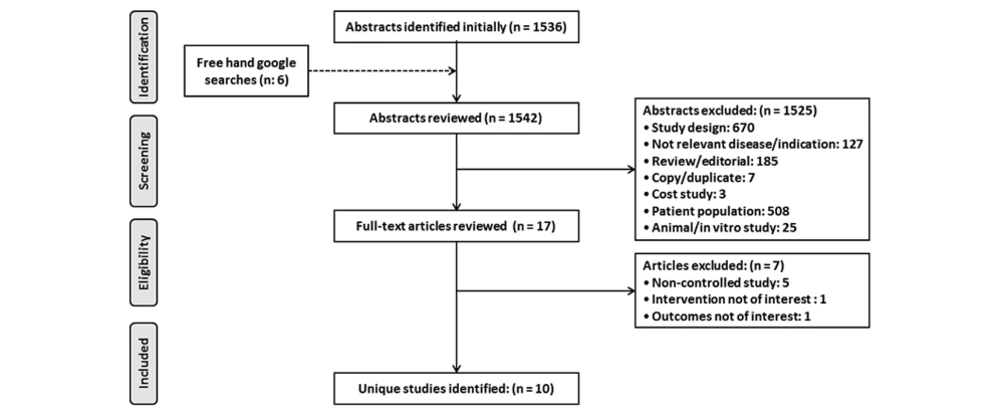
Running the searches in the electronic databases yielded 1542 results in total (Figure 1). After duplicates were removed and reports were screened by title, keywords and abstract, they were assessed for inclusion and exclusion criteria. A full-article review of the remaining 17 identified articles was necessary to determine the eligibility for this review. Based on the full article review, 10 articles received a full evaluation for qualitative and quantitative analysis. The primary findings from the included studies are summarized in Table 1.
| Authors, Year of Publication, Country | Study Design | Characteristics of Participants | Intervention and Sample Size (Analyzed) | Study Duration, mo | Measured Outcome (P Values) | Authors’ Conclusions | ||
|---|---|---|---|---|---|---|---|---|
| Sample Size | Cisplatin Dose | Antioxidant Group | Placebo Group | |||||
| Benoehr et al., 2005, Germany (17) | R, P, SB, PC | 36 | 50 mg/m2 | Theophylline (350 mg, three times daily), 4 days | Placebo | - | S. Cr (P < 0.001), eGFR | Prophylactic application of theophylline as i.v. loading dose and oral maintenance regimen may preserve kidney function in CIN |
| El-Ghiaty et al., 2014, Egypt (18) | R, P, O | 49 | 70 mg/m2 | Cystone (225 mg, two tablets thrice daily), 18 weeks | Placebo | 23 | S. Cr (P < 0.001), Cr. Cl (P < 0.001) | Cystone could protect from CIN |
| Ghorbani et al., 2013, Iran (19) | R, DB | 122 | 203.72 mg | Selenium (400 mcg tablet), 1 week | Placebo | - | ICIN (P = 0.013) | Selenium could probably prevent CIN along with hydration therapy |
| Hemati et al., 2012, Iran (20) | R, PC | 46 | 76 mg/m2 | Vitamin E (400 IU) + Selenium (200 μg) | Placebo | 16 | eGFR (P < 0.001) | Vitamin E and selenium could be used to reduce CIN |
| Karademir et al., 2016 (25) | R, PC | 64 | 50 mg/m2 | Theophylline (400 mg, oral), 5 days | Placebo | 36 | SCr (P = 0.965), GFR (P = 0.149) | Less nephrotoxicity developed in theophylline as compared to placebo |
| Momeni et al., 2015, Iran (21) | R, PC | 60 | - | Silymarin (140 mg/bid tablet), 7 days | Placebo | - | S. Cr (P = 0.001), BUN | Silymarin could decrease CIN |
| Mousavi et al., 2014, Iran (26) | R, P, DB, PC | 76 | 50 mg/m2 | Aminophylline (4 mg/kg, i.v.) + theophylline (200 mg, three times daily, p.o.), 4 days | Placebo | 4 | ICIN | Prophylactic application of aminophylline and theophylline did not have a protective effect against CIN |
| Shahbazi et al., 2015, Iran (27) | R, DB, PC | 30 | 185 - 220 mg | Silymarin (420 mg) | Placebo | 12 | eGFR (P = 0.01), S. Cr | Prophylactic silymarin treatment could not prevent CIN |
| Smyth et al., 1997, Multi-country (22) | R, DB, PC | 151 | 100 mg/m2 | Glutathione (3 g/m2), 3 weeks for 6 courses | Placebo | - | ICIN (P = 0.006) | Glutathione improved CIN patient's quality of life |
| Weijl et al., 2004, The Netherlands (28) | R, DB, PC | 50 | 70 mg/m2 | Antioxidant micronutrients (Vitamin C (1000 mg) + Vitamin E (400 mg) + Selenium (100 μg)) | Placebo | 30 | Cr. Cl (P < 0.05) | Antioxidant treatment could not prevent CIN due to poor compliance and/or inadequate supplementation |
Characteristics of Included Studies (N = 9)
4.2. Characteristics of the Studies
4.2.1. Included Studies
Table 1 shows the study characteristics. There were 672 enrolled patients in total, of whom 330 (49.10%) patients received antioxidant treatment whereas the remaining patients were on placebo or conventional treatment. Out of 12 randomized controlled trials, five were double-blinded (19, 22, 26-28), one was single-blinded (17), and one was open-controlled (18).
The potential of various antioxidant treatments against CIN was assessed by measuring changes in BUN, eGFR, serum creatinine, creatinine clearance, and CIN incidence. The change in BUN was measured in two studies (18, 21), change in eGFR in four studies (17, 20, 25, 27), change in serum creatinine in five studies (17, 18, 21, 25, 27), change in creatinine clearance in two studies (18, 28), and the incidence of CIN in four studies (19, 22, 26, 27).
4.2.2. Excluded Studies
Seven studies were excluded from this review. Five excluded studies were non-controlled studies, one study did not report any separate outcome for CIN patients, and one study did not use any antioxidant for intervention.
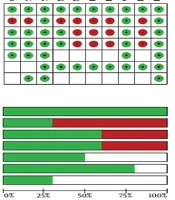
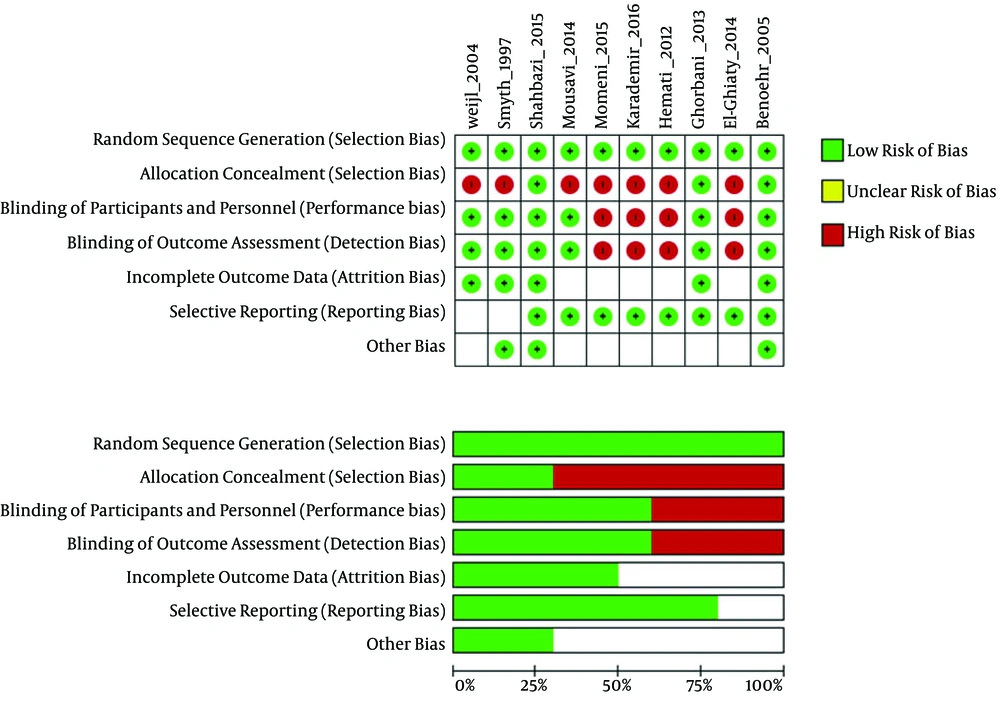
4.3. Risk of Bias
The results of the risk of bias (ROB) assessment for the included studies are presented in Figure 2. The included studies showed high variations in overall quality. The randomized studies included in this review varied in their study design, structure, and methodology. Of 10 studies identified for analysis in this study, five were classified as high-quality (17, 19, 22, 27, 28) and five as low-quality (18, 20, 21, 25, 26).
Downs and Black scoring was used to evaluate the quality of the studies. The quality of the studies was variable. The overall quality of study reporting was good, external validity was low, and internal validity was good amongst studies (Table 2). The heat map of the overview of the quality of studies determined using Downs and Black scoring system is provided in Appendix 1 in Supplementary File.
| Authors, Year of Publication | Reporting (Max: 11) | External Validity (Max: 3) | Internal Validity-Bias (Max: 7) | Internal Validity-Confounding (Selection Bias) (Max: 6) | Total (27) |
|---|---|---|---|---|---|
| Benoehr et al., 2005 (17) | 10 | 3 | 5 | 5 | 23 |
| El-Ghiaty et al., 2014 (18) | 9 | 3 | 3 | 4 | 19 |
| Ghorbani et al., 2013 (19) | 8 | 3 | 5 | 5 | 21 |
| Hemati et al., 2012 (20) | 8 | 3 | 3 | 4 | 18 |
| Karademir et al., 2016 (25) | 8 | 3 | 3 | 4 | 18 |
| Momeni et al., 2015 (21) | 8 | 3 | 3 | 4 | 18 |
| Mousavi et al., 2014, Iran (26) | 8 | 3 | 5 | 3 | 19 |
| Shahbazi et al., 2015 (27) | 11 | 3 | 6 | 6 | 26 |
| Smyth et al., 1997 (22) | 8 | 3 | 5 | 4 | 20 |
| Weijl et al., 2004 (28) | 9 | 3 | 7 | 5 | 24 |
Quality of Studies Based on the Downs and Black Scoring System
4.4. Heterogeneity
There was significant heterogeneity between the studies in the patient population, nephrotoxicity definition, and duration of antioxidant treatment. Therefore, the random-effects model was required in all analyses.
Individual and cumulative cisplatin dose varied considerably among the studies (Table 1). In the studies on human participants, individual cisplatin dose ranged from 50 to 100 mg/m2 of the body surface area.
4.5. Conflicting Evidence
An array of research studies evaluated the efficacy and safety of antioxidants for the treatment of CIN, but with conflicting results. Studies by Benoehr et al. (2005) and Karademir et al. (2016) found that theophylline treatment preserved kidney function in CIN (17, 25) whereas Mousavi et al. (2014) reported that theophylline did not have a protective effect against CIN (26). Momeni et al. (2015) reported that silymarin could decrease the progression of CIN (21); however, Shahbazi et al. (2015) reported that silymarin treatment could not prevent CIN (27). As low statistical power may limit the interpretability of the findings, the results of these trials should be interpreted with caution.
4.6. Outcomes
Because of heterogeneity in the studies, we used a random-effects model for the analysis of changes (Δ) in BUN, eGFR, serum creatinine, and creatinine clearance whereas a fixed-effects model was used for the analysis of the incidence of CIN (Table 3).
| Outcomes | Heterogeneity | Analysis Model | Summary Statistics | Overall Effect | No. Trials | ||
|---|---|---|---|---|---|---|---|
| I2 (%) | P Value | SMD/OR [95% CI] | P Value | ||||
| Serum creatinine, mg/dL | 96 | < 0.00001 | Random | SMD | -3.40 [-5.47, -1.33] | 0.001 | 5 |
| BUN, mg/dL | 94 | < 0.0001 | Random | SMD | -5.96 [-10.07, -1.86] | 0.004 | 2 |
| Creatinine clearance, mL/min | 98 | < 0.00001 | Random | SMD | -2.50 [-7.67, 2.67] | 0.34 | 2 |
| eGFR, mL/min | 96 | < 0.00001 | Random | SMD | -3.77 [-6.16, -1.38] | 0.002 | 4 |
| Incidence of CIN | 0 | 0.43 | Fixed | OR | 0.37 [0.11, 1.29] | 0.12 | 4 |
Clinical Outcomes of Antioxidant Groups Compared to Control Groups
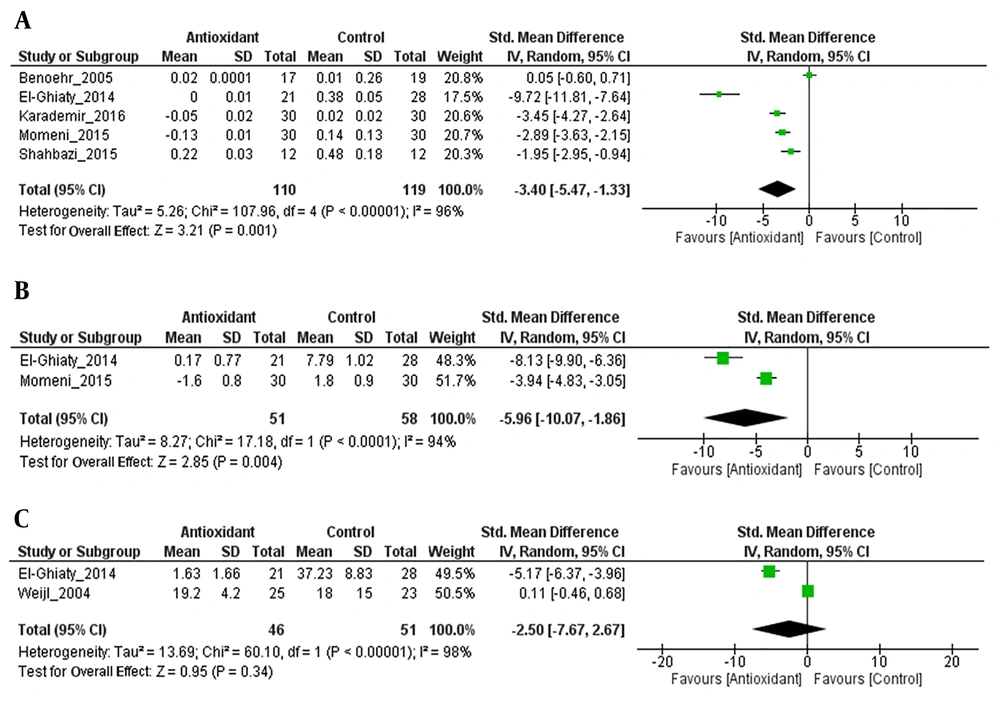
Antioxidant treatment showed a significant reduction in serum creatinine (Z = 3.21; P = 0.001) and there was heterogeneity between the studies (I2 = 96%; P < 0.00001) (Figure 3A).
As shown in Figure 3B, antioxidant treatment resulted in a significant change in BUN, with an overall effect size (Z) of 2.85; however, there was heterogeneity between the studies (I2 = 94%; P < 0.0001). The change in BUN was significantly higher in the antioxidant group than in the control group (SMD = -5.96, 95% CI: -10.07 to -1.86, P = 0.004).
The intervention with antioxidants was associated with a non-significant change in creatinine clearance levels (Z = 0.95; P = 0.34), with substantial heterogeneity between the studies (I2 = 98%; P < 0.00001). The change in creatinine clearance levels was more in the antioxidant group than in the control group (SMD = -2.50, 95% CI: -7.67 to 2.67; P = 0.05) (Figure 3C). However, the meta-analysis was considered inappropriate because of the small number of studies.
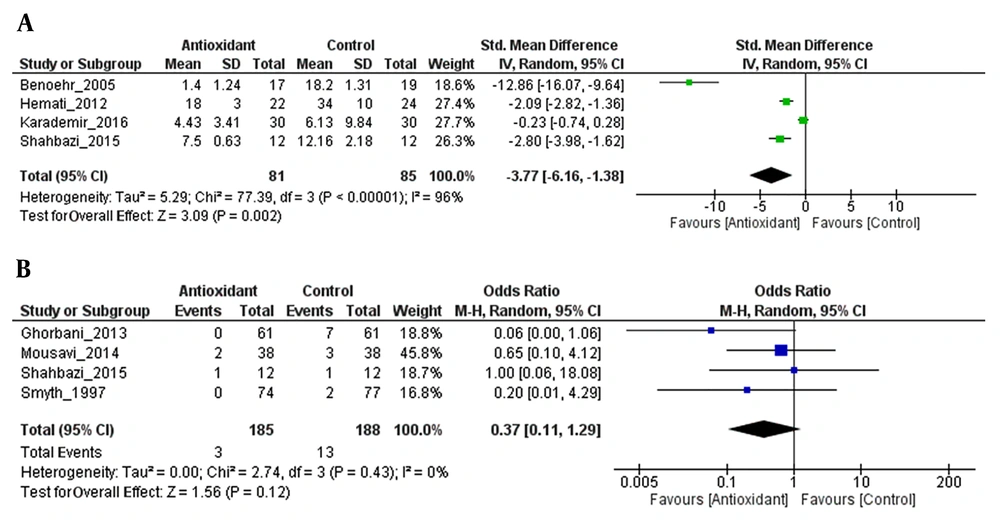
Antioxidant combination resulted in a significant change in eGFR, with an overall effect size (Z) of 3.09 (P = 0.002) and with substantial heterogeneity (I2 = 96%; P = 0.00001). The change in eGFR was significantly more in the antioxidant group than in the control group (SMD = -3.77, 95% CI: -6.16 to -1.38; P = 0.002) (Figure 4A).
The efficacy of antioxidant treatment in the reduction of CIN incidence lost its statistical significance (Z = 1.56; P = 0.12). There was no heterogeneity between the studies (I2 = 0%; P = 0.43). There was no statistically significant difference in the incidence of CIN between the antioxidant group and the control group (SMD = 0.37, 95% CI: 0.11 to 1.29; P = 0.12) (Figure 4B).
5. Discussion
Nowadays, cisplatin (CDDP) is a well-known and widely used antineoplastic agent in the treatment of a wide range of malignancies. However, it causes renal impairment and induces toxicity in approximately 30% - 40% of treated patients (5). The cisplatin-induced nephrotoxicity (CIN) leads to decreased glomerular filtration rate (GFR), altered creatinine clearance, impaired urinary albumin excretion ratio, increased serum creatinine (S. Cr), and elevated blood urea nitrogen (BUN) level (29, 30). Thus, it is essential to attenuate the acute manifestations and renal failure along with subclinical reduced kidney function. A recent systematic review was conducted to examine the efficacy of hydration therapy in the prevention or amelioration of CIN (9). However, the findings of the reviewed studies showed that hydration therapy was useful only in patients receiving high-dose CDDP and in some patients, over-diuresis resulted in dehydration (9). Thus, currently, no ideal nephroprotective agent exists in clinical use for CIN treatment.
Some clinical evidence supports the use of antioxidant supplements in the prevention of CIN (17-22). Furthermore, in most countries, many herbal supplements with antioxidant potential are used under the name of functional food, medicinal food, or food supplement for the treatment of various diseases including CIN. However, we need to determine the efficacy and safety of these herbal supplements used in the treatment of CIN. Hence, the current systematic review and meta-analysis study was undertaken to examine the randomized controlled trials that directly evaluated the role of various antioxidants against CIN.
A good number of the popularly used cisplatin for its efficacy against various malignancies and it has been well documented that its use is associated with a significantly higher risk of acute renal injury (31). Various clinical studies suggested that the administration of antioxidants significantly decreases the incidence of CIN (19, 22, 26, 27). We identified 10 eligible studies, including 672 participants, and found that antioxidant treatment was effective in the amelioration of kidney function altered by the administration of cisplatin in cancer patients. The results of the present meta-analysis also showed similar findings indicating that antioxidant treatment ameliorates the incidence of CIN (OR: 0.37 [0.11, 1.29], P = 0.12).
The altered serum creatinine level is one of the important clinical features of CIN that has been consistently observed in the available body of evidence (18, 32). It has been well documented that the increased level of ROS induces mesangial cells contraction and modifies the filtration surface area, resulting in an increased serum creatinine level (33, 34). Previous preclinical studies suggested that antioxidant supplementation inhibits free radical damage and thereby decreases GFR and finally inhibits CIN-induced elevated serum creatinine (35, 36). Our meta-analysis indicated that the administration of antioxidants significantly decreased the cisplatin-induced increase in GFR (SMD = -3.77, 95% CI: -6.16 to -1.38; P = 0.002) and serum creatinine (SMD = -3.40, 95% CI: -5.47 to -1.33; P = 0.001). The findings of the present meta-analysis are in line with those of in-vivo animal studies where antioxidant treatment decreased serum creatinine levels (37). Contrary to the findings of our review, limited clinical evidence shows that the administration of antioxidants such as silymarin does not produce any significant change in GFR and serum creatinine concentration (27). However, this conflicting result could be explained based on several factors. First, preclinical studies might not represent the biological processes clinically (38). Second, the duration of treatment might be insufficient to exert its effect, as Shahbazi et al. (2015) reported no significant effect of silymarin when administered 48 h before the initiation of cisplatin infusion (27) whereas a report by Momeni et al. (2015) showed that the prophylactic administration of silymarin for seven days prevented CIN (21). Thus, the beneficial effects of antioxidant supplements against CIN might be related to the timing of their administration.
Earlier studies suggested that creatinine clearance and BUN are important clinical factors that usually predict nephrotoxicity (39) and serve as confounding factors for the selection of dose and schedule of cisplatin during treatment of malignancies in cancer patients (40, 41). In most trials, patients treated with cisplatin showed increased creatinine clearance, which is a major concern regarding CIN (41). Antioxidants have shown beneficial effects against CIN in preclinical studies (35, 36); however, they have failed to show benefits under clinical circumstances (28). The results of the present meta-analysis indicate that there is a discrepancy in the results of animal studies and those of randomized controlled trials concerning the association between antioxidants and amelioration in creatinine clearance. The non-significant decrease in creatinine clearance by antioxidant treatment in CIN may be related to poor compliance (28). However, our findings are similar to those of a previous meta-analysis where hydration therapy showed significant attenuation in creatinine clearance, which is essential for patients to prevent CIN (9).
5.1. Limitation
Although our meta-analysis suggested a possible clinical benefit for the antioxidant treatment of CIN, it had important limitations, the comparators varied too much, and the effects were inconsistent and imprecise. As limitations, first, we determined the efficacy of antioxidant supplements that were synthetic in nature (except for that used by El-Ghiaty et al. (2004)); thus, the findings of the present meta-analysis cannot be extended to antioxidants that are derived or extracted from plants. Second, there was a large statistical heterogeneity due to the lack of patient selection in the present study. Third, the baseline characteristics such as tumor type, CDDP dose, and nephrotoxicity definition varied largely across the studies, which limits our ability to make concrete recommendations regarding the implication of antioxidant supplements in CIN. Fourth, since the majority of the patient populations enrolled in studies were male, the findings of the present meta-analysis are applicable to male patients, which may result in gender bias. Furthermore, preclinical studies show that CIN more frequently occurs in male rats than in female rats, which may be due to the higher expression level of OCT2 (organic cation transporter 2) in males than in females (42). However, conflicting results exist possibly related to the risk factors of CIN and gender difference (31, 43). Thus, there is a need for studies that enroll populations with equal gender distribution in the future. Finally, the publication bias could not be excluded from the present meta-analysis, as six studies were published positively among the 10 included studies.
6. Conclusions
In conclusion, the findings of this systematic review and meta-analysis suggest that antioxidant treatment is associated with the reduced risk of CIN. The findings of the present investigation have important clinical implications where antioxidant treatment can be used in patients with CIN who do not respond to other therapies such as hydration, diuresis, or magnesium supplementation. However, future studies concerning the comparative effectiveness of antioxidants for preventing CIN need to stratify patients according to baseline characteristics. More research could strengthen the evidence of the efficacy of antioxidant treatment in patients with high CIN risk.