1. Background
Plants are considered as important sources of new chemical compounds that can be used in the development of novel therapeutic drugs (1). Drugs obtained from plants can present a variety of biological activities, including antioxidant activity (2). When cellular antioxidant defenses become overwhelmed by reactive oxygen and nitrogen species (RONS), oxidation of lipids, proteins, and DNA may occur, resulting in cellular damage (3). In humans, oxidative stress has been shown to be involved in many diseases, such as cancer (4), diabetes mellitus (5), Parkinson’s (6) and Alzheimer’s (7) diseases, atherosclerosis (8), and rheumatoid arthritis (9).
Antioxidants play a significant protective role against oxidative stress at the cellular level. Antioxidant defense systems may be enzymatic or non-enzymatic, and both work together to ensure the redox balance of the cells (10, 11). In addition to antioxidant enzymes, plant cells also have a variety of natural antioxidants, such as phenolic compounds derived from secondary metabolism (12). Antioxidants are important in the preservation of food lipids (13), and due to safety concerns about the use of synthetic antioxidants, natural antioxidants obtained primarily from plant material, have attracted increased interest (14).
Phragmanthera glaucocarpa (Peyr.) Balle belongs to the Loranthaceae family, which is comprised of 950 species, divided to 77 genera (15). Plants in this family are hemi-parasites on shrubs and wild and cultivated trees (16). They depend on their host to obtain water and nutrients, and the damage caused to the host plant is variable (17). The plants belonging to the Loranthaceae family have various applications in traditional medicine, for example, the species Struthanthus concinnus, Loranthus micranthus and Viscum album are used in the treatment of tuberculosis, cancer and cardiovascular diseases, respectively (18, 19). A recent study on the roots of Phragmanthera glaucocarpa demonstrated that a chloroform fraction contains compounds capable of inhibiting the enzyme acetylcholinesterase (20). In the literature, no studies were found about the antioxidant activity of the roots of Phragmanthera glaucocarpa.
2. Objectives
The aim of the present study was to evaluate the in vitro antioxidant activity of the entire extract and four fractions were obtained from the roots of this medicinal plant, used in Angola folk medicine.
3. Methods
3.1. Reagents, Chemicals and Equipment
Chloroform, ethyl acetate, methanol, and toluene were purchased from Carlo Erba reagents group (France). ABTS (2,2’-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid), DPPH (1’,1’-diphenyl-2-picrylhydrazyl), Folin-Ciocalteu’s phenol reagent, gallic acid monohydrate, potassium persulfate, potassium iodide, starch, and trolox were purchased from Sigma-Aldrich (Portugal). Acetic acid was purchased from Riedel-de Haën (Germany). Ethanol was purchased from Manuel Vieira & Cª (Irmão) Lda (Portugal). n-Hexane was purchased from Valente e Ribeira Lda (Portugal). Sodium carbonate was purchased from Fluka (Switzerland). Sodium thiosulfate was purchased from Merck (Portugal). The sunflower oil was purchased from the local supermarket (Covilhã, Portugal). All reagents were used without further purification, except for n-hexane, which was purified by distillation through a solvent distiller apparatus (Winkler TST-II). UV-Vis measurements were performed using an Evolution 160 spectrophotometer from Thermo-scientific (USA). A rotary evaporator (VV 2000; Heidolph, Germany) was used for solvent evaporation and concentration of all plant extracts. A block heater from Stuart (USA) was used in the peroxide value method to cause thermal oxidation of the samples.
3.2. Plant Selection and Extract Preparation
Medicinal plant selection was based on indigenous ethnopharmacological records. The roots of Phragmanthera glaucocarpa were collected in Angola, in the province of Uíge, and a voucher specimen (n°2074) was deposited and identified by the staff of Luanda herbarium. After collection, the roots were dried in the dark at room temperature and then powdered and extracted with methanol for a week at room temperature, yielding the methanolic extract. The methanolic extract was fractioned into n-hexane, toluene, chloroform, ethyl acetate, and aqueous fractions. All fractions and the original extract were concentrated using a rotary evaporator at 40°C and stored at 4°C for further use.
3.3. Antioxidant Activity
3.3.1. ABTS Radical-Scavenging Activity
The ABTS (2,2’-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) radical scavenging assay was performed according to the method described by Re et al. (21) with slight modifications. ABTS●+ radical cations were generated by reacting 5 mL of 7 mM ABTS with 88 µL of 140 mM potassium persulfate. The reaction mixture was allowed to stand in the dark for 16 hours at room temperature and was used within one day. ABTS●+ working solution was prepared by diluting the stock solution described above with 50% ethanol to an absorbance of 0.700 ± 0.050 at 734 nm. Measurements were taken from a mixture of 3 mL of ABTS●+ solution and 30 µL of different concentrations of methanolic extract solution (0.025 - 50 mg/mL) over a period of 15 minutes, and each set of measurements was taken at least three times and the experiment was performed in triplicates. The reaction took place directly in the measuring cuvette, and 3030 µL of methanol was used as a blank to set zero absorbance. Trolox was used as a reference in the same way as the extract solutions. The radical scavenging (RS) (%) was Based on Equation 1:
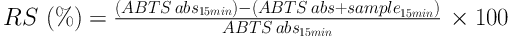
Where ABTS abs15min is the absorbance of the ABTS●+ solution with 30 µL of methanol (control) and ABTS abs + sample15min is the absorbance of the ABTS●+ solution with 30 µL of the extract or reference sample (t = 15 minutes). The sample concentration (mg/mL), providing 50% of initial ABTS radical scavenging activity (EC50), was calculated from the curve where radical scavenging (%) was plotted versus the extract concentration (mg/mL).
3.3.2. 1,1-Diphenyl-2-Picrylhydrazyl Radical Scavenging Activity
The DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay was performed according to the method reported by Villano et al. (22) with minor modifications. Briefly, 200 µL of methanol (control) or methanolic extract solutions (0.025 to 50 mg/mL) were added to 2.7 mL of 40 µM DPPH methanolic solution. Mixtures were shaken, and the absorbance at 515 nm was measured using a UV-Vis spectrophotometer over a period of 15 minutes. Antioxidant capacity, expressed as percentage of DPPH radical scavenging after 15 minutes, was calculated using the same equation as the ABTS assay. The antioxidant capacity of the tested samples and the trolox (reference) for the DPPH radical was expressed as EC50 (mg/mL) and calculated as indicated above for the ABTS assay.
3.3.3. Total Phenolics
The total phenolic compound content of the extracts was determined using the Folin-Ciocalteu reagent, according to the method reported by Matkowski and Piotrowska (23), with minor modifications. A total of 400 µL of a 0.05 mg/mL methanolic extract solution was reacted with 2 mL of Folin-Ciocalteu reagent (0.2 M) for eight minutes at room temperature. After that, 1600 µL of 7.5% (w/v) sodium carbonate was added, and the mixture was allowed to stand for 90 minutes at room temperature. Absorbance was measured at 765 nm with a UV-Vis spectrophotometer, and the total phenolics quantification was performed based on a standard curve with gallic acid as the standard compound. The absorbance versus concentration curve could be described by the equation y = 10.199x + 0.005 (R2 = 0.999), in which y is the absorbance and x is the concentration. The results were expressed as milligrams of gallic acid equivalents (G.A.E.) per gram of dry extract.
3.3.4. Peroxide Value Assay
Sunflower oil was used as the lipidic model to determine the effect of the different extracts on lipid oxidation. Initially, the number of days required to achieve a peroxide value of approximately 100 meq O2/g of lipid sample for the sunflower oil was determined. In a glass tube with a stopper, 22 mL of oil was added and then heated to 80°C in the absence of light. During the oxidation time, the peroxide value of the sunflower oil was determined, according to the Cd 8 - 53 method of the Official Methods and Recommended Practices of the American Oil Chemists’ Society (24) with minor modifications. Briefly, 2.5 g of sample was swirled in 15 mL of a 3:2 acetic acid-chloroform solution until dissolved. At this point, 0.25 mL of a saturated potassium iodide (KI) solution was added, and the mixture was shaken for one minute. Then, 15 mL of distilled water was added to the mixture, followed by 0.25 mL of starch solution 1% (w/v). The final mixture was then titrated with 0.1 M sodium thiosulfate standardized with a fresh solution of I3-, prepared from KIO3 plus KI. The peroxide value (PV) was calculated according to the Equation 2.
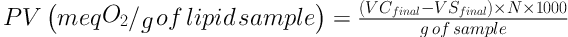
Where N is the normality of sodium thiosulfate solution used, and VCfinal and VSfinal are the volumes of sodium thiosulfate solution spent on the titration of the control and sample, respectively. Eight days of heating was required to achieve a peroxide value of approximately 100 meq O2/g of lipid sample for the sunflower oil. The peroxide value for the various extract solutions was determined using the same procedure as for the sunflower oil. The sample to be oxidized composed of a homogeneous mixture of 20 mL of sunflower oil and 2 mL of a 10 µg/mL methanolic extract solution. The sample composed of the homogeneous mixture of 20 mL of sunflower oil and 2 mL of methanol was used as a control.
3.4. Statistical Analysis
All determinations were conducted in triplicates for each test sample, and the results were presented with mean ± standard deviation (SD). Sigmaplot release 11.0 software (Systat Software Inc., USA) was used to make the figures, calculate the EC50 values by linear regression analysis, and perform statistical calculations. One-way Analysis of Variance (ANOVA) and Tukey’s multiple comparison tests were used to determine significance. P < 0.05 was considered statistically significant.
4. Results and Discussion
4.1. Medicinal Plant Extracts
The extract and fractions obtained from the roots of Phragmanthera glaucocarpa and used to evaluate the antioxidant activity and total phenolic content are listed in Table 1. For each sample a numerical code was assigned.
| Solvent | Number |
|---|---|
| Hexane | 1 |
| Methanol | 2 |
| Ethyl acetate | 3 |
| Chloroform | 4 |
| Water | 5 |
Extract and Fractions of Phragmanthera glaucocarpa Roots Used in the Evaluation of Antioxidant Activity
4.2. Antioxidant Activity by ABTS and DPPH Methods
The ABTS and DPPH methods were used to evaluate the potential antioxidant activity of the extract and fractions obtained from the roots of Phragmanthera glaucocarpa (Table 1). In these methods, the ability of the extracts to scavenge ABTS and DPPH radicals was measured and expressed as EC50 value in mg/mL (mean ± SD). The EC50 represents the concentration of tested samples that can scavenge half of the ABTS or DPPH radicals. All plant extracts showed a propensity to quench the ABTS and DPPH free radicals, as indicated by a decrease in absorbance in the presence of the plant extracts. For the ABTS method, trolox was used as the reference compound, and the EC50 value found in the literature regarding the radical ABTS ranged from 2.00 × 10-3 mg/mL (25) to 0.03 mg/mL (26). The EC50 value obtained for trolox in relation to ABTS●+ was 3.00 × 10-3 ± 1.00 × 10-4 mg/mL (Figure 1). Considering the EC50 obtained for trolox in relation to ABTS●+, Figure 1 shows that all samples presented lower ABTS●+ scavenging activity compared to trolox. Of the five samples, only the ethyl acetate fraction (sample 3) presented good capacity to scavenge ABTS radicals, with an EC50 value of 1.06 ± 0.30 mg/mL.
EC50 for the different Phragmanthera glaucocarpa (Table 1) and Trolox samples with the ABTS method. 1- Hexane fraction; 2- methanol extract; 3- ethyl acetate fraction; 4- chloroform fraction and 5- water fraction. Means ± SD (n = 3).
Regarding the DPPH method, as for ABTS, trolox was also used as a reference compound, and the EC50 value found in the literature ranged from 5.00 × 10-3 mg/mL (25) to 6.00 × 10-3 mg/mL (27). The EC50 obtained for trolox relative to DPPH● was 5.04×10-3 ± 1.00×10-4 mg/mL (Figure 2). Through the data presented in Figure 2, and taking into consideration the EC50 value obtained for trolox in relation to DPPH radicals, all samples presented less DPPH● scavenging activity compared to trolox. Of the five samples, only methanol (sample 2) and aqueous (sample 5) extracts presented good capacity to scavenge DPPH radicals, with an EC50 value of 0.10 ± 0.01 mg/mL and 0.10 ± 0.00 mg/mL, respectively. Of the five samples, sample 3 presented good reactivity with ABTS radicals and sample 2 and 5 had good reactivity with DPPH radicals. However, the chemical composition of these three samples is not known, and thus the separation and identification of the compounds, as well as the realization of new tests with the isolated compounds, is crucial to determine which compound or compounds are responsible for scavenging ABTS and DPPH radicals. Further experiments with isolated compounds is important because the isolated compounds may lead to better results, or, on the contrary, reduction of the reactivity with ABTS●+ and DPPH● may occur due to possible synergistic effects between the different compounds present in the extracts.
EC50 for the Phragmanthera glaucocarpa (Table 1) and Trolox samples with the DPPH method. 1- Hexane fraction; 2- methanol extract; 3- ethyl acetate fraction; 4- chloroform fraction and 5- water fraction. Means ± SD (n = 3).
4.3. Total Phenolic Content
Quantification of total phenolics was performed using the standard curve described in 3.3.3, and the results are presented in Figure 3. The total phenolic concentration ranged from 34.41 mg G.A.E./g of dry extract to 280.42 mg G.A.E./g of dry extract. From the five extract samples of Phragmanthera glaucocarpa, the ethyl acetate fraction (sample 3) showed the highest total phenolic content with 280.42 ± 0.15 mg G.A.E./g of dry extract. The lowest phenolic content was found in the aqueous fraction (sample 5) with 34.41 ± 0.91 mg G.A.E. /g of dry extract. Generally, it is assumed that higher concentration of total phenolics is related to higher antioxidant capacity of the extract (27).
Total phenolic content of the different Phragmanthera glaucocarpa samples (Table 1) determined by the Folin-Ciocalteu method. 1- Hexane fraction; 2- methanol extract; 3- ethyl acetate fraction; 4- chloroform fraction and 5- water fraction. Means ± SD (n = 3).
Comparing the results obtained using the methods of ABTS (Figure 1) and DPPH (Figure 2) with the results obtained for the Folin-Ciocalteu (Figure 3), it was observed that the ethyl acetate fraction (sample 3) presented the highest phenolic content and good capacity to scavenge ABTS radicals. In relation to DPPH, the methanol extract (sample 2) and aqueous fraction (sample 5) presented good capacities to scavenge DPPH radicals and a low phenolic content. Although the ABTS and DPPH methods share the same reaction mechanism, as both are single electron transfer (SET) assays (28), there are some important differences among these two methods, which may explain the different results obtained for the five samples. In fact, ABTS and DPPH radicals may be neutralized either by direct reduction via single electron transfers or by radical quenching via hydrogen atom transfer (HAT; (29)). Thus, ABTS and DPPH radicals can react with different compounds present in the extracts through different reaction mechanisms and yield different results. Another reason that may explain the different results is the solvent used for the solubilization of the DPPH and ABTS radicals. For the DPPH radicals, methanol was used as the solvent, whereas for ABTS radicals, a mixture of ethanol and water (50:50, v/v) was used. Some authors found better antioxidant activity results, using the ethanol and water mixture (30). Additionally, DPPH radicals are more suitable for application in hydrophobic systems, while ABTS radicals can be used in both hydrophilic and lipophilic systems and thus react with a wider variety of compounds than DPPH radicals (31).
4.4. Peroxide Value (PV)
The peroxide value of a lipidic sample in the presence of the five fractions was determined using the Cd 8 - 53 method. The peroxide value results of sunflower oil in the presence of the five samples are presented in Figure 4. The value obtained for control on the eighth day of oxidation was 265.00 ± 3.43 meq O2/g of the lipid sample (Figure 4). Samples with a peroxide value above the peroxide value of the control have an oxidizing effect on the sunflower oil. Figure 4 shows that the peroxide value ranged from 512.40 meq O2/g of lipid sample to 131.40 meq O2/g of lipid sample.
Peroxide values for sunflower oil in the presence of the five Phragmanthera glaucocarpa samples (Table 1) and for the control after eight days of lipidic oxidation. 1- Hexane fraction; 2- methanol extract; 3- ethyl acetate fraction; 4- chloroform fraction and 5- water fraction. Means ± SD (n = 3). **** Indicate P < 0.0001, which denotes the statistical comparison between the control and the various extracts. “ns” indicates not statistically significant differences.
Analyzing Figure 4 for different samples and compared with the result obtained for the control, it was observed that the greatest reduction of the peroxide value on the eighth day of sunflower oil oxidation was obtained with the aqueous fraction (sample 5), with 131.40 ± 1.05 meq O2/g of lipid sample, and the hexane fraction (sample 1) with 183.420 ± 1.06 meq O2/g of lipid. Extract samples 3 and 4 had a pro-oxidant effect in the sunflower oil. Sample 2 showed no statistically significant difference compared to the control. Comparing the results obtained for the peroxide value and the EC50 value in the ABTS and DPPH methods, it was concluded that the aqueous fraction of Phragmanthera glaucocarpa (sample 5), despite the low reactivity with ABTS●+ and a low concentration of total phenolic compounds, contains compounds with good reactivity with DPPH● and also presented sufficient results in the reduction of the peroxide value of sunflower oil. The ethyl acetate fraction presented higher phenolic content and also good capacity to scavenge ABTS radicals, however, the peroxide value was high, which indicates that the compounds present in this extract have a pro-oxidant effect on sunflower oil. The same oxidizing effect on sunflower oil was observed for the chloroform fraction. The hexane fraction only presented good results in the reduction of the peroxide value of the sunflower oil. The methanol extract, despite having good reactivity with DPPH radicals, presented a low concentration of total phenolic compounds, a low capacity in scavenging ABTS radicals, and a low reduction in the peroxide value of the sunflower oil.
5. Conclusions
The results of the present study indicate that the methanol extract and the ethyl acetate and aqueous fraction from the roots of Phragmanthera glaucocarpa contain chemical compounds with significant radical scavenging activity. The results also suggest that the antioxidant activity of Phragmanthera glaucocarpa might be due to the presence of phenolics and other phytochemical constituents present in the extracts. Based on the obtained results, Phragmanthera glaucocarpa also contains compounds with the ability to reduce the oxidation of lipid samples, which might have applications in food and pharmaceutical industries. Since the chemical composition of the extract of Phragmanthera glaucocarpa is not known, further investigations need to be carried out with isolated, purified, and identified compounds because purified compounds may lead to better results. Additionally, further in vivo studies need to be assessed for future clinical uses. Considering the results obtained with the extract and fractions from the roots of Phragmanthera glaucocarpa, it can be concluded that this medicinal plant can be regarded as a promising source of natural compounds, particularly phenolic compounds with antioxidant activity.



