1. Context
The Scutellaria genus that can be found in East Asia, the United States, and Europe include perennial flowering plants in the Lamiaceae (mint) family, which contains 350 species (1, 2). There are about 300 species of this genus in Asia (3-6). In Iran, the Scutellaria genus is represented by 27 species, which 12 of them are endemic (7). Scutellaria genus has been used for the treatment of hyperlipidemia, allergy, inflammatory, arteriosclerosis, hepatitis, and hypertension for hundreds of years (8). It’s about 2000 years that Asian medicine, especially Chinese medicine, is using Scutellaria for the treatment of fevers, colds, diphtheria, and high blood pressure (9). The genus of Scutellaria has several therapeutic properties such as antitumor, hepatoprotective, antioxidant, anti-inflammatory, anticonvulsant, antibacterial, and antiviruses activities (8). Also, Scutellaria species are useful in treating nervous system problems, including anxiety, insomnia, and hysteria (2). More than 295 compounds have been isolated from this genus (10, 11), such as flavonoids (12), phenylethanoid glycosides, and terpenes (Iridoid glycosides, monoterpenes, diterpenes, and triterpenoids) (13). Flavonoids (almost flavones) are common bioactive compounds of the Scutellaria genus (14).
Essential oils are secondary metabolites with versatile organic structures that have useful medicinal properties (15). They can be extracted from plants using classical and advanced techniques (16). There are various methods to extract essential oils, such as hydrodistillation, steam distillation, microwave, organic solvent extraction, supercritical CO2, and ultrasonic and high-pressure solvent extraction (17, 18). Various factors affect the compositions of the essential oils, including geographical and climatic conditions, harvesting time, physiological age, plant storages, extraction methods, kind of drying. Besides, it should be noted that various parts of the plant contain different compositions (19-22). The major essential oil compounds are terpenoids, which are classified as monoterpens, sesquiterpenes, diterpenes, triterpenes, and tetraterpenes (23, 24). Essential oils can defend the plant against predators, parasites, environmental stress, and diseases. Meanwhile, during the insects’ reproductive process, they attract insects (25).
2. Evidence Acquisition
The recent literature about the essential oils of different species of Scutellaria was reviewed (26-32). Although some review studies are conducted on the Scutellaria genus (8, 13) but evidence about the Scutellaria essential oils is not sufficient. Therefore this review was focused on the chemical compounds and biological activities of the Scutellaria genus essential oils.
3. Results
3.1. Chemical Compositions of Scutellaria Genus Essential Oils
Many studies have shown variations in the chemical compounds of Scutellaria genus essential oils. Several factors affect the variations in the essential oil compositions, including harvesting time, soil PH, drying conditions, geographic location, kind of subspecies, part of the plant, and extraction method (19-22).
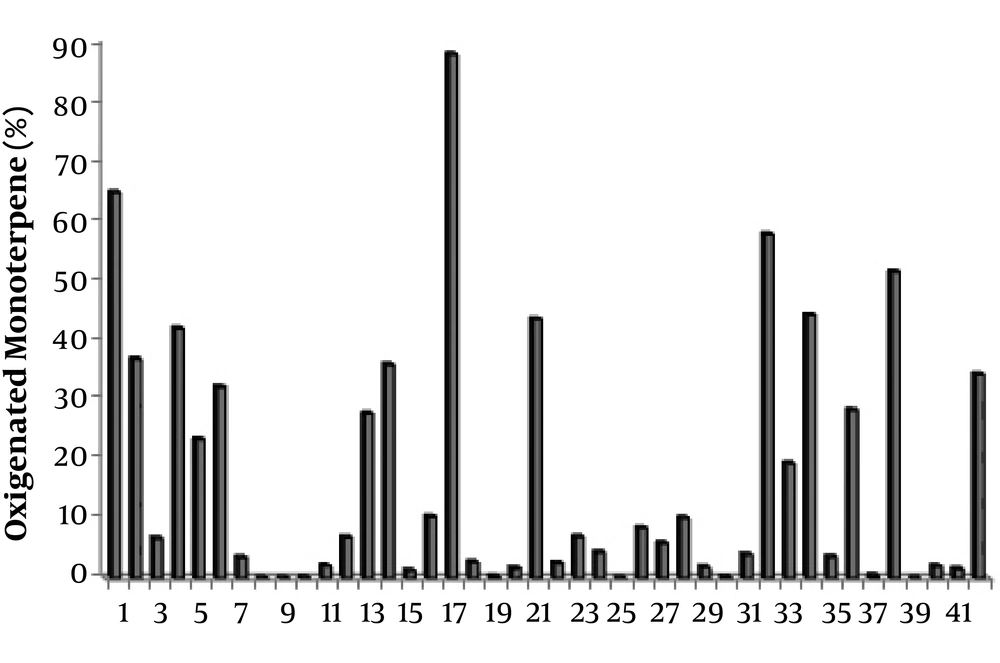
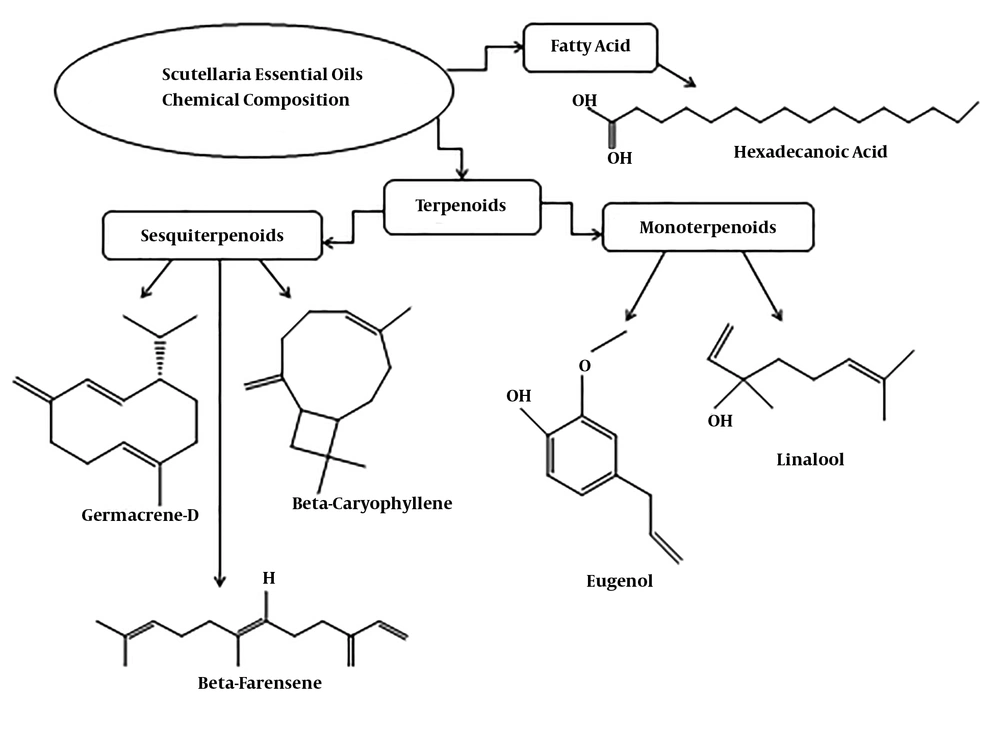
The oils extracted from different Scutellaria species have similar compounds (Table 1). The compositions of essential oils are classified in Figures 1 to 4; the sesquiterpenes are the most common compound of the Scutellaria essential oils. Hexadecanoic acid, Germacrene D, β-caryophyllene, Linalool, β-Farnesene, and Eugenol are the main compounds of the essential oils of this genus. The structures of these compounds are shown in Figure 5. Several hydrocarbons and oxygenated terpenoid compounds have been identified from Scutellaria species (Figures 2 to 5). Hexadecanoic acid is a saturated fatty acid in plants, animals, and microorganisms (33). Germacrene D is a precursor of various sesquiterpenes such as cadinenes and selinenes (34, 35). Germacrene D has insecticidal activity against mosquitoes (36), aphids (37), and ticks (38). β-caryophyllene is a natural sesquiterpene with dietary phytocannabinoid that has therapeutic potential for anxiety, neuropathic pain, ulcerative colitis, endometriosis, and renal protection (39-42). Linalool is a monoterpene compound that is found in many plants; it is effective against several bacteria and fungi and possesses anti-inflammatory, antinociceptive, and antihyperalgesic activities (43). β-Farnesene is a strong pheromone in most aphid species (44).
| Compound | Scutellaria species | Origin | Amount (%) | Ref. |
|---|---|---|---|---|
| Hexadecanoic acid | S. barbata | Korea | 58.52 | (45) |
| S. albida subsp albida | Turkey | 15.6 | (46) | |
| S. albida subsp colchica | Turkey | 12.9 | (46) | |
| S. albida subsp velenovskyi | Turkey | 17.3 | (46) | |
| S. brevibracteata | Lebanon | 12.6 | (47) | |
| S. diffusa | Turkey | 29.9 | (48) | |
| S. heterophylla | Turkey | 16.0 | (48) | |
| S. barbata | China | 28.6 | (49) | |
| Hydroxynaphthalene | S. barbata | Korea | 12.22 | (45) |
| Germacrene D | S. volubilis | Ecuador | 20.4 | (50) |
| S. baicalensis | United States | 12.4 | (51) | |
| United States | 27.5 | (51) | ||
| United States | 13.0 | (51) | ||
| S. litwinowii | Iran | 16.9 | (52) | |
| S. strigillosa | China | 37.78 | (53) | |
| S. salviifolia | Turkey | 40.0 | (46) | |
| S. laeteviolacea | Japan | 21.67 | (54) | |
| S. orientalis subsp alpina | Iran | 39.7 | (55) | |
| S. orientalis subsp Virens | Iran | 16.5 | (56) | |
| S. ramosissima | Uzbekistan | 23.96 | (57) | |
| S. sibthorpii | Turkey | 42.01 | (58) | |
| S. heterophylla | Turkey | 21 | (46) | |
| S. pinnatifida subsp alpina | Iran | 39.7 | (55) | |
| β -Caryophyllene | S. volubilis | Ecuador | 17.5 | (50) |
| S. baicalensis | US | 22.3 | (51) | |
| S. baicalensis | US | 23.1 | (51) | |
| S. baicalensis | US | 41.5 | (51) | |
| S. californica | US | 56.6 | (51) | |
| S. albida subsp albida | Turkey | 14.2 | (48) | |
| S. albida subsp velenovskyi | Turkey | 20 | (48) | |
| S. sieberi | Greece | 14.2 | (28) | |
| S. salviifolia | Turkey | 11 | (46) | |
| S. orientalis subsp alpina | Iran | 15 | (55) | |
| S. orientalis subsp Virens | Iran | 13.4 | (56) | |
| S. orientalis subsp Virens | Turkey | 22.08 | (59) | |
| S. ramosissima | Uzbekistan | 11.09 | (57) | |
| S. sibthorpii | Turkey | 22.58 | (58) | |
| S. brevibracteata | Lebanon | 14.4 | (47) | |
| S. hastifolia | Lithuania | 12.9 | (47) | |
| S. hastifolia | Lithuania | 12.9 | (60) | |
| S. galericulata | Canada | 29.4 | (61) | |
| S. heterophylla | Turkey | 13.0 | (46) | |
| S. pinnatifida subsp. alpina | Iran | 15.0 | (55) | |
| S. rubicunda | Italy | 28.7 | (26) | |
| S. luteo-caerulea | Iran | 24.8 | (62) | |
| S. parvula | Canada | 29.4 | (61) | |
| S. havanensis Jacq. | Cuba | 75.6 | (63) | |
| α-Humulene | S. volubilis | Ecuador | 14.7 | (50) |
| S. havanensis Jacq. | Cuba | 11.6 | (63) | |
| Linalool | S. albida subsp albida | Turkey | 20.4 | (46) |
| S. albida subsp condensata | Turkey | 28.5 | (46) | |
| S. albida subsp albida | Greece | 52.6 | (27) | |
| S. sieberi | Greece | 22.7 | (28) | |
| S. rupestris | Greece | 38.8 | (28) | |
| S. schachristanica | Uzbekistan | 26.98 | (57) | |
| S. cypria var. elatior | Turkey | 10.92 | (58) | |
| S. rubicunda | Italy | 27.8 | (26) | |
| Nerolidol | S. albida subsp condensata | Turkey | 16.8 | (46) |
| Tetradecanoic acid | S. albida subsp velenovskyi | Turkey | 10.2 | (46) |
| Cadinene | S. lateriflora | Iran | 27.0 | (64) |
| S. orientalis subsp virens | Turkey | 19.92 | (59) | |
| Calamenene | S. lateriflora | Iran | 15.2 | (64) |
| β-Farnesene | S. litwinowii | Iran | 20.3 | (52) |
| S. galericulata | Canada | 17.0 | (61) | |
| S. parvula | Canada | 17.0 | (61) | |
| S.Wightiana benth | India | 22.07 | (13) | |
| Bicyclo-germacrene | S. salviifolia | Turkey | 14.0 | (46) |
| Hexahydro farnesyl acetone | S. orientalis subsp. alpina | Lebanon | 11.7 | (47) |
| 1-octen-3-ol | S. laeteviolacea | Japan | 27.72 | (54) |
| S. grossa Wall ex Benth | India | 32.0 | (65) | |
| Terpinolene | S. orientalis subsp Virens | Iran | 15.6 | (56) |
| Acetophenone | S. immaculata | Uzbekistan | 30.39 | (57) |
| S. schachristanica | Uzbekistan | 34.74 | (57) | |
| Eugenol | S. immaculata | Uzbekistan | 20.61 | (57) |
| S. schachristanica | Uzbekistan | 20.67 | (57) | |
| S. cypria var cypria | Turkey | 23.05 | (58) | |
| Thymol | S. immaculata | Uzbekistan | 10.04 | (57) |
| Palmitic acid | S. cypria var cypria | Turkey | 27.0 | (57) |
| S. cypria var elatior | Turkey | 46.76 | (57) | |
| Phytol | S. brevibracteata | Lebanon | 10.7 | (47) |
| 4-vinylguaiacol | S. brevibracteata | Lebanon | 10.2 | (47) |
| βisabolol | S. galericulata | Canada | 20.6 | (61) |
| S. parvula | Canada | 20.6 | (61) | |
| Bergamotene | S. galericulata | Canada | 13.4 | (61) |
| S. parvula | Canada | 13.4 | (61) | |
| Methyl chavicol | S. pinnatifida A. Hamilt Subsp pinnatifida | Iran | 81.9 | (66) |
| Aromadendrene | S. repens | India | 30.7 | (67) |
| β -Funebrene | S. repens | India | 15.0 | (67) |
| 1, 4- Benzenediol-2, 5-dimethyl | S.Wightiana benth | India | 21.53 | (13) |
| Pipertone oxide | S.Wightiana benth | India | 16.23 | (13) |
| α-Humulene | S. havanensis Jacq | Cuba | 11.6 | (63) |
| Limonene | S. angustifolia | Laos | 30.3 | (68) |
| Fenchone | S. angustifolia | Laos | 26.7 | (68) |
| Alpha-pinene | S. angustifolia | Laos | 11.9 | (68) |
Major Essential Oil Components (> 10%) of Scutellaria Species
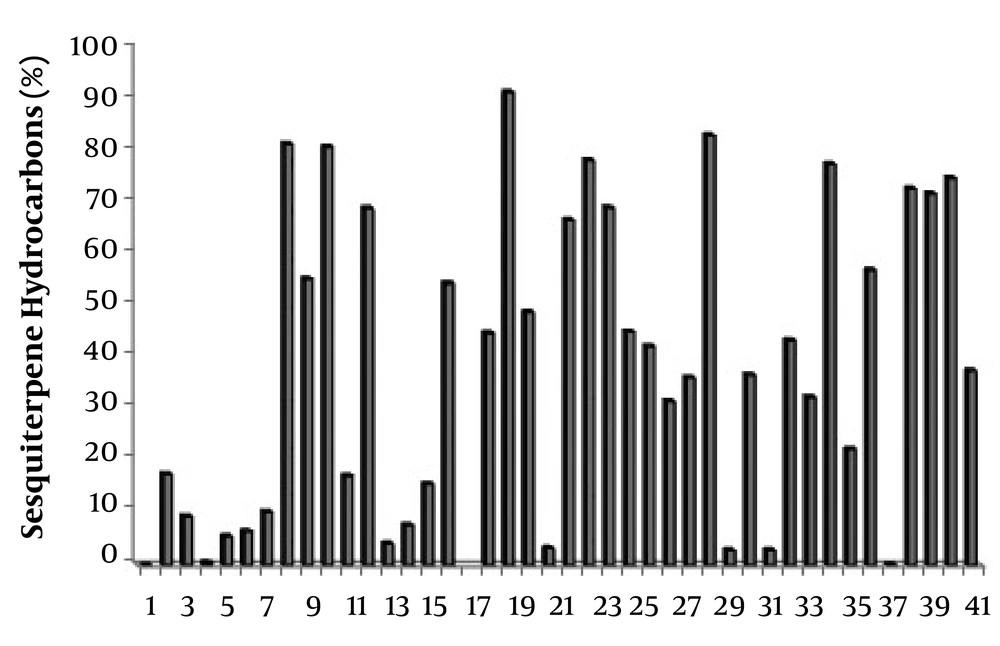
Relative abundance of sesquiterpene hydrocarbons in the essential oils of Scutellaria species. 1: S. Albida (27); 2: S. albida subsp albida (47); 3: S. albida subsp colchica (47); 4: S. albida subsp condensata (47); 5: S. albida subsp velenovskyi (47); 6: S. barbata (69); 7: S. barbata (50); 8: S. baicalensis (Chinese Medicinal Plants) (52); 9: S. baicalensis (UC Berkeley Botanical Gardens) (52); 10: S. baicalensis (Horizon Herbs) (52); 11: S. brevibracteata (48); 12: S. californica (60); 13: S. cypria var. elatior (59); 14: S. cypria var. Cypria (59); 15: S. diffusa (49); 16: S. galericulata (63); 17: S. grossa Wall ex Benth (67); 18: S. hastifolia (48); 19: S. havanensis Jacq (65); 20: S. heterophylla (49); 21: S. immaculate (58); 22: S. orientalis L. subsp. Virens (61); 23: S. lateriflora (64); 24: S. litwinowii (53); 25: S. laeteviolacea (55); 26: S. luteo-caerulea (64); 27: S. orientalis ssp. alpina (48); 28: S. orientalis subsp. Virens (57); 29: S. pinnatifida ssp. alpina (56); 30: S. pinnatifida ssp. alpina (68); 31: S. parvula (63); 32: S. rupestris (28); 33: S. ramosissima (58); 34: S. rubicunda (26); 35: S. repens (67); 36: S. sieberi (28); 37: S. strigillosa (54); 38: S. schachristanica (58); 39: S. sibthorpii (59); 40: S. salviifolia (49); 41: S. volubilis (51); 42: S. Wightiana Beth (13).
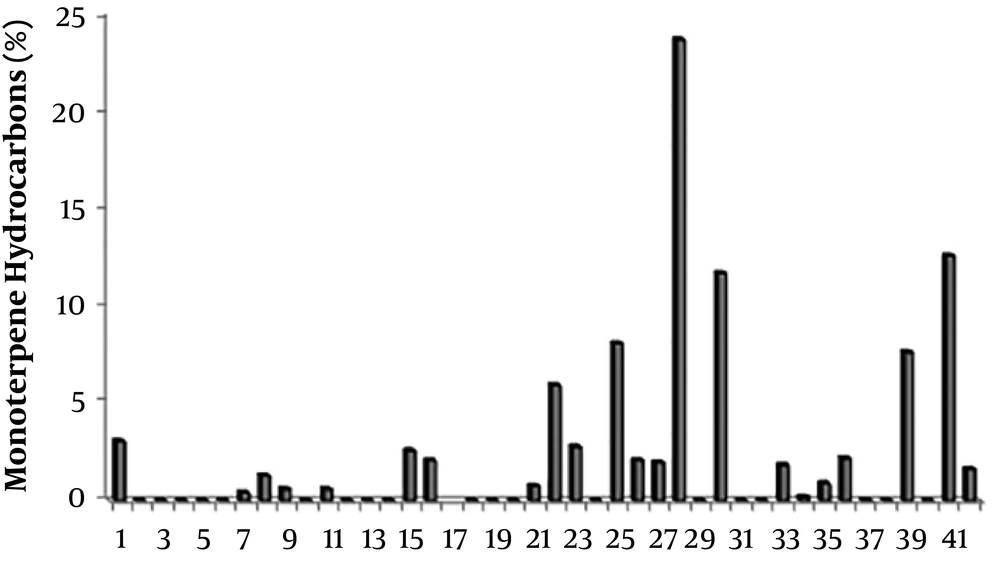
Relative abundance of monoterpene hydrocarbons in the essential oils of Scutellaria species. 1: S. Albida (27); 2: S. albida subsp albida (47); 3: S. albida subsp colchica (47); 4: S. albida subsp condensata (47); 5: S. albida subsp velenovskyi (47); 6: S. barbata (69); 7: S. barbata (50); 8: S. baicalensis (Chinese Medicinal Plants) (52); 9: S. baicalensis (UC Berkeley Botanical Gardens) (52); 10: S. baicalensis (Horizon Herbs) (52); 11: S. brevibracteata (48); 12: S. californica (60); 13: S. cypria var. elatior (59); 14: S. cypria var. Cypria (59); 15: S. diffusa (49); 16: S. galericulata (63); 17: S. grossa Wall ex Benth (67); 18: S. hastifolia (48); 19: S. havanensis Jacq (65); 20: S. heterophylla (49); 21: S. immaculate (58); 22: S. orientalis L. subsp. Virens (61); 23: S. lateriflora (64); 24: S. litwinowii (53); 25: S. laeteviolacea (55); 26: S. luteo-caerulea (64); 27: S. orientalis ssp. alpina (48); 28: S. orientalis subsp. Virens (57); 29: S. pinnatifida ssp. alpina (56); 30: S. pinnatifida ssp. alpina (68); 31: S. parvula (63); 32: S. rupestris (28); 33: S. ramosissima (58); 34: S. rubicunda (26); 35: S. repens (67); 36: S. sieberi (28); 37: S. strigillosa (54); 38: S. schachristanica (58); 39: S. sibthorpii (59); 40: S. salviifolia (49); 41: S. volubilis (51); 42: S. Wightiana Beth (13).
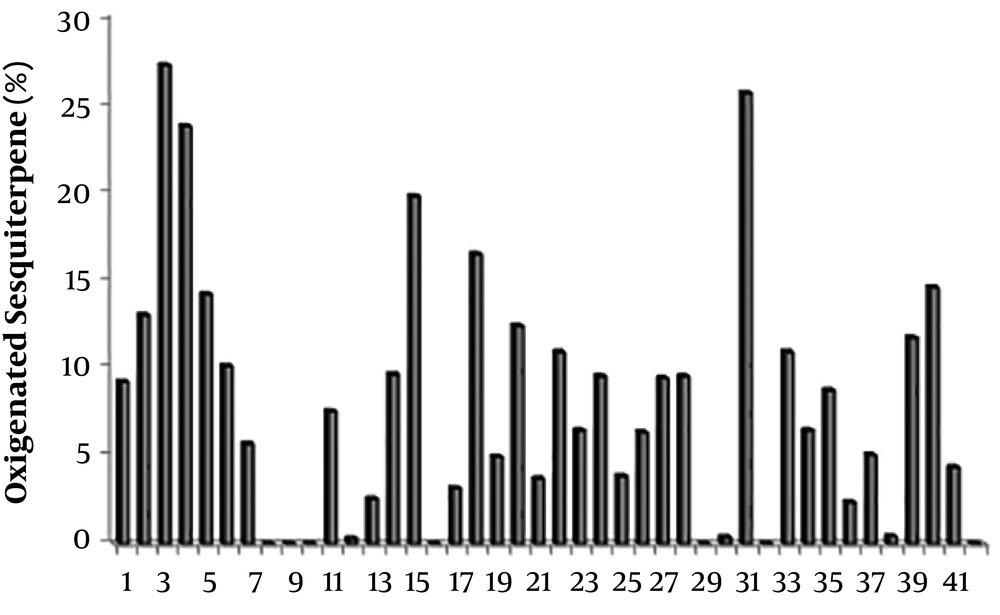
Relative abundance of oxygenated sesquiterpenes in the essential oils of Scutellaria species. 1: S. Albida (27); 2: S. albida subsp albida (47); 3: S. albida subsp colchica (47); 4: S. albida subsp condensata (47); 5: S. albida subsp velenovskyi (47); 6: S. barbata (69); 7: S. barbata (50); 8: S. baicalensis (Chinese Medicinal Plants) (52); 9: S. baicalensis (UC Berkeley Botanical Gardens) (52); 10: S. baicalensis (Horizon Herbs) (52); 11: S. brevibracteata (48); 12: S. californica (60); 13: S. cypria var. elatior (59); 14: S. cypria var. Cypria (59); 15: S. diffusa (49); 16: S. galericulata (63); 17: S. grossa Wall ex Benth (67); 18: S. hastifolia (48); 19: S. havanensis Jacq (65); 20: S. heterophylla (49); 21: S. immaculate (58); 22: S. orientalis L. subsp. Virens (61); 23: S. lateriflora (64); 24: S. litwinowii (53); 25: S. laeteviolacea (55); 26: S. luteo-caerulea (64); 27: S. orientalis ssp. alpina (48); 28: S. orientalis subsp. Virens (57); 29: S. pinnatifida ssp. alpina (56); 30: S. pinnatifida ssp. alpina (68); 31: S. parvula (63); 32: S. rupestris (28); 33: S. ramosissima (58); 34: S. rubicunda (26); 35: S. repens (67); 36: S. sieberi (28); 37: S. strigillosa (54); 38: S. schachristanica (58); 39: S. sibthorpii (59); 40: S. salviifolia (49); 41: S. volubilis (51); 42: S. Wightiana Beth (13).
Relative abundance of oxygenated monoterpenes in the essential oils of Scutellaria species. 1: S. Albida (27); 2: S. albida subsp albida (47); 3: S. albida subsp colchica (47); 4: S. albida subsp condensata (47); 5: S. albida subsp velenovskyi (47); 6: S. barbata (69); 7: S. barbata (50); 8: S. baicalensis (Chinese Medicinal Plants) (52); 9: S. baicalensis (UC Berkeley Botanical Gardens) (52); 10: S. baicalensis (Horizon Herbs) (52); 11: S. brevibracteata (48); 12: S. californica (60); 13: S. cypria var. elatior (59); 14: S. cypria var. Cypria (59); 15: S. diffusa (49); 16: S. galericulata (63); 17: S. grossa Wall ex Benth (67); 18: S. hastifolia (48); 19: S. havanensis Jacq (65); 20: S. heterophylla (49); 21: S. immaculate (58); 22: S. orientalis L. subsp. Virens (61); 23: S. lateriflora (64); 24: S. litwinowii (53); 25: S. laeteviolacea (55); 26: S. luteo-caerulea (64); 27: S. orientalis ssp. alpina (48); 28: S. orientalis subsp. Virens (57); 29: S. pinnatifida ssp. alpina (56); 30: S. pinnatifida ssp. alpina (68); 31: S. parvula (63); 32: S. rupestris (28); 33: S. ramosissima (58); 34: S. rubicunda (26); 35: S. repens (67); 36: S. sieberi (28); 37: S. strigillosa (54); 38: S. schachristanica (58); 39: S. sibthorpii (59); 40: S. salviifolia (49); 41: S. volubilis (51); 42: S. Wightiana Beth (13).
Despite the many reports about essential oils of Scutellaria species (more than 38), a large number of species have not been studied yet. Therefore, more studies on the chemical composition of unstudied Scutellaria essential oils are recommended.
3.2. Biological Activities of Scutellaria Essential Oils
3.2.1. Antioxidant Activity
Compared to the results on the antioxidant activity of Scutellaria extracts (70-72), the essential oils of Scutellaria species only have moderate antioxidant activity (57). Zokirjonovna et al. (2016) evaluated the antioxidant activity of essential oils of three Uzbek Scutellaria species (i.e., S. immaculata, S. ramosissima, and S. schachristanica). The Scutellaria essential oils of these species exhibited moderate antioxidant activity due to the presence of eugenol, thymol, and carvacrol, but it was weaker than ascorbic acid (57).
3.2.2. Antimicrobial Activity
There are reports on the biological activities of Scutellaria genus essential oils, and most of the studies have investigated the antimicrobial activity of essential oils from this genus. The antimicrobial activity of these oils could be due to the components such as linalool, eugenol, and other long-chain alcohols (73). Moreover, other compounds such as thymol and alpha-terpineol could also contribute to the antimicrobial activity of the essential oil (74, 75). Yu et al. (2004) investigated the antibacterial activities of S. barbata essential oils against 17 microorganisms (Enterococcus faecalis, Staphylococcus aureus, Serratia marcescens, Escherichia coli, Stenotrophomonas maltophila, Pseudomonas aeruginosa, Staphylococcus heamolyticus, Staphylococcus epidermidis, Candida tropicalis, Staphylococcus simulans, Citrobacter freundii, Salmonella paratyphi-A, Shigella flexneri, Klebsiella pneumoniae, Salmonella typhi, Serratia liquefaciens, and Candida albicans) using the disc diffusion and broth microdilution methods. According to their results, the essential oil demonstrated a strong bactericidal effect; S. epidermidis was the most sensitive microorganism (29 mm inhibition zone and 0.77 mg/mL MBC), and C. albicans was the most resistant to the extract (7 - 9 mm and 24.50 mg/mL MBC) (69). Based on the results reported by Zhu et al. (2016), the essential oils from S. strigillosa had higher antimicrobial effects on gram-positive bacteria than gram-negative bacteria and fungus (53). Another study by Pant et al. (2012) demonstrated that the essential oils of S. grossa had significant antibacterial activity against B. subtilis, E. faecalis, K. pneumonia, and S. enterica (65). Skaltsa (2005) reported a moderate activity against S. aureus and B. cereus for the essential oils of S. rupestris and S. sieberi that were collected from Greece (28). In a study by Skaltsa et al. (2000), it was revealed that the essential oil of S. albida subsp albida was moderately active against E. coli, S. aureus, B. subtilis, P. aeruginosa, and S. cerevisiae, which can be attributed to high levels of linalool and nerolidol content (27). Dereboylu et al. (2012) investigated the antimicrobial activities of the volatile compounds of S. sibthorpii, S. cypria var. cypria, and S. cypria var. elatior against 7 bacteria and one fungis (S. aureus, B. subtilis, S. typhimurium, E. faecalis, E. coli, P. aeruginosa, K. pneumonia, and C. albicans) and reported that S. aureus was the most sensitive microorganism (58). The antibacterial activity of S. repens essential oil was tested on S. aureus, E. faecalis, A. tumefac iens, E. chrysanthemi, X. phaseoli, E. coli, S. enterica, K. pneumoniae, and P. multocida (67), and according to the results, the essential oil showed a high level of antibacterial activity. The maximum zone of inhibition was 23 mm for E. coli, 18 mm for E. faecalis, 15 mm for K. pneumonia, and 12 mm for B. subtilis (67). The antimicrobial activities of Scutellaria essential oils are summarized in Table 2.
| Microorganism | Scutellaria Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. barbata | S. strigillosa | S. grossa | S. rupestris ssp. adenotricha | S. sieberi | S. albida ssp. albida | S. cypria var. cypria | S. sibthorpis | S. repens | |
| S. aureus | √ | √ | -a | √ | √ | √ | √ | √ | √ |
| E. coli | √ | √ | - | - | - | √ | √ | √ | √ |
| P. aeruginosa | √ | √ | - | - | - | √ | √ | - | - |
| S. epidermidis | √ | - | - | - | - | - | - | - | - |
| S. heamolyticus | √ | - | - | - | - | - | - | - | - |
| S. simulans | √ | - | - | - | - | - | - | - | - |
| E. faecalis | √ | - | √ | - | - | - | √ | - | √ |
| C. freundii | √ | - | - | - | - | - | - | - | - |
| K. pneumoniae | √ | - | √ | - | - | - | √ | √ | √ |
| S. flexneri | √ | - | - | - | - | - | - | - | - |
| S. paratyphi | - | - | - | - | - | - | - | - | - |
| S. liquefaciens | √ | - | - | - | - | - | - | - | - |
| S. marcescens | √ | - | - | - | - | - | - | - | - |
| S. maltophilia | √ | - | - | - | - | - | - | - | - |
| C. albicans | √ | √ | - | - | - | - | - | - | - |
| C. tropicalis | √ | - | - | - | - | - | - | - | - |
| B. subtilis | - | √ | √ | - | - | - | √ | √ | √ |
| S. cerevisiae | - | √ | - | - | - | √ | - | - | - |
| S.enterica | - | - | √ | - | - | - | - | - | √ |
| B. cereus | - | - | - | √ | √ | √ | - | - | - |
| M. flavus | - | - | - | - | - | - | - | - | - |
| P. mirabilis | - | - | - | - | - | - | - | - | - |
| S. thyphimirium | - | - | - | - | - | - | √ | √ | - |
| X. phaseoli | - | - | - | - | - | - | - | - | √ |
| E.chrysanthemi | - | - | - | - | - | - | - | - | √ |
| A.tumefaciens | - | - | - | - | - | - | - | - | √ |
| P. multocida | - | - | - | - | - | - | - | - | √ |
Antibacterial and Antifungal Activities of Scutellaria Species
3.2.3. Antifeedant Activity
In the study performed by Formisano et al. (2013), the essential oils of three Scutellaria species (S. brevibracteata, S. hastifolia and S. orientalis ssp. alpina) are studied against the feeding and egg-laying behavior of Spodoptera littoralis. The results of the insect assays showed that the essential oil of S. hastifolia was the only oil that could deter Spodoptera littoralis larvae from feeding on treated discs, whereas both S. brevibracteata and S. hastifolia could deter female moths from laying eggs on papers treated with their extracts (47). In another study, Rosselli et al. (2007) reported that essential oil of S. rubicunda subsp. linnaeana has antifeedant activity against Spodoptera littoralis (26). In their study, the essential oil of plant stimulated a dose-dependent positive feeding response from larvae of S. littoralis (feeding index (FI) 50% = 925 ppm; FI at 100 ppm = 44.85). A study on S. rubicunda subsp. linnaeana revealed that aerial parts of the plant that contains scutecyprol B, scutalbin C, and scutecyprol B had antifeedant activity against larvae of five species of Lepidoptera (FI at 100 ppm = 100) (1).
3.2.4. Phytotoxic Effect
The phytotoxic effect of S. strigillosa essential oil was evaluated by conducting bioassays against amaranth and bluegrass (amaranthus is a cosmopolitan genus of annual or short-lived perennial plants, and bluegrass refers to several species of grasses of the genus Poa). 3 μL/mL of essential oil could completely inhibit amaranthus seedling growth and caused a significant inhibitory effect on bluegrass (53).
3.2.5. Acaricidal Toxicities Activity
The acaricidal activity of S. barbata essential oil was higher than the activity observed in the positive controls (benzyl benzoate), which was evaluated via fumigant and contact toxicity bioassays against Dermatophagoides farinae, D. pteronyssinus, and Tyrophagus putrescentiae (45).
4. Conclusions
Scutellaria is a genus in the Lamiaceae family and for thousands of years, has been used as a medicine (76, 77). In recent years, many studies are performed on the essential oils of different species of Scutellaria (8, 13, 26-32). However, many species of the Scutellaria genus are not investigated, and therefore many studies can be performed on the components and biological activities of uninvestigated Scutellaria essential oils.
In the current review, chemical compositions of essential oils and biological activities (antioxidant, antimicrobial, antifeedant, phytotoxic, and acaricidal activities) of the Scutellaria genus are summarized. Hexadecanoic acid, germacrene D, β-caryophyllene, linalool, β-farnesene, and eugenol were the main compounds. (several compounds of these oils have medicinal properties). This review can serve as a reference for natural products and ethnopharmacology fields.