1. Background
Cisplatin (CP) is a chemotherapy drug widely used to treat cancer. Clinical studies reported nephrotoxicity induced by CP (1). Acute kidney injury is observed in 25% - 35% of patients receiving cisplatin (2). In the kidney of patients receiving cisplatin, concentration of CP in epithelial cells of proximal tubule is about five times higher than that of the serum value (3). Lower CP concentrations induce apoptosis through caspase activations, while induce necrosis in higher concentrations (4). CP with generation of reactive oxygen species (ROS) and destroying cell components induces nephrotoxicity (5). Kidney removes most of the harmful substances produced in the body or entered from outside such as chemotherapy drugs. Therefore, kidneys, as one of the vital organs, clean blood and maintain the chemical balance in the body. This organ is the main target of drug-induced damage that accumulates in large quantities in the epithelial cells of nephrons (6). Studies indicated that acute and chronic CP treatments cause kidney damage and increase the risk of renal failure (7). Increased serum levels of creatinine and blood urea nitrogen (BUN) are the side effects of cisplatin, which changes renal function (2). The mechanisms of kidney damage caused by cisplatin are generation of free radicals, mitochondrial dysfunction, and apoptosis (8). Also, oxidative stress by alterations in redox homeostasis directly damages renal parenchyma and leads to impaired renal microvascular activity and subsequently renal dysfunction (9).
Zataria multiflora Boiss (ZM) (Avishan-e-Shirazi), a member of Lamianceae family, is one of the largest families of herbal plants growing all around the world. ZM is a medicinal herb used to treat various diseases. Pharmacological properties of ZM including anti-inflammatory (10), radioprotective (11), chemoprotective (12), antibacterial, and antioxidant effects (13) are reported in the numerous studies. Thymol and carvacrol, as phenolic compounds, are the main active components of ZM (14). Studies show that thymol by its antioxidant and anti-inflammatory properties can suppress lipid peroxidation and subsequently protect healthy organs against toxicity induced by oxidative stress (15) and chemotherapy (16). Also, some studies confirmed the protective effect of thymol against nephrotoxicity in mice (17).
2. Objectives
The current study aimed at assessing the protective effect of ZM against cisplatin-induced nephrotoxicity via biochemical, histopathological, and immunohistochemical assessments.
3. Methods
3.1. Plant Material and Extraction
Dried aerial parts of ZM were collected in the flowering season from Firozabad county in Fars province, Iran. Homogenous powder was macerated in 70% ethanol for 72 hours (1:10 w/v), after which the hydro alcoholic extract of dried ZM was processed by removing the solvent using a rotary (Heidolph, Germany). The ZM extract was standardized based on thymol as the major active ingredient using RP-HPLC (reverse-phase high-performance liquid chromatography) method according to the authors’ previous reports (18, 19).
3.2. Experimental Animals
Thirty-two adult male BALB/c mice (25 - 30 g) were used in the study. The mice were obtained from Animal Research Center of Mazandaran University of Medical Sciences, Sari, Iran and kept under suitable conditions (23 ± 2°C, 55% ± 5% humidity, 12-hour dark/light cycle). A standard pellet chow and fresh tap water were available ad libitum.
3.3. Study Design
In the experimental study, the mice were randomly divided into four groups (eight animals per group): (I) received normal saline, (II) received 200 mg/kg ZM daily for seven consecutive days via oral gavage, (III) received a single dose of CP (10 mg/kg) intraperitoneally on the 5th day of study, and (IV) received ZM + CP. Doses of CP and ZM were chosen according to previous studies (12, 20).
3.4. Specimen Collections
One day after the last treatment, the animals were anesthetized with ketamine and xylazine. Blood samples were collected from the heart. Serum samples were used to measure creatinine and urea levels. Then, animals were sacrificed, one kidney of each animal was immediately removed and fixed in 10% buffer formalin for histological and immunohistochemistry (IHC) assay. And the other one stored at -80°C for biochemical analysis.
3.5. Biochemical Analysis
The testicular lipid peroxidation was determined by measuring the concentration of malondialdehyde (MDA) using thiobarbituric acid through spectrophotometry. MDA content was expressed as nM/mg protein. Tetramethoxypropane (TEP) was used as the standard specimen.
The protein carbonyl (PC) was assessed spectrophotometrically by reading the absorption rate at a wavelength of 365 nm with an absorption coefficient of 22,000 M-1cm-1 and expressed as a nmol of DNPH per milligram of protein.
Content of the glutathione (GSH) in the samples was determined by spectrophotometer with 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) as an indicator at 412 nm and expressed as µM.
3.6. Serum Markers of Kidney Damage
The serum creatinine and BUN levels were determined using Pars Azmoon kits, Iran, (cat. No. creatinine: 1-400-009 and BUN: 1-400-029).
3.7. Histological Assay
For histopathological assay and to determine the effect of CP and ZM on kidney, samples were fixed in 10% (w/v) buffer formalin for 24 hours. After processing and embedding, samples were cut into 5-µm thick sections stained with periodic acid-Schiff (PAS). Degeneration, infiltration of leukocytes, atrophy of tubular and glomeruli, detachment of tubular epithelial cell, pyknosis of renal epithelium nuclei, and the appearance of protein columna were scored. As a final point, tubular damage (5 field/ section) was scored based on the percentage of cortical tubules showing epithelial necrosis: 0 index; < 25% damage scored 1; 25% - 50% damage scored 2; 50% - 75% damage scored 3, and > 75% damage scored 4 (21). All the histological studies were evaluated by a histologist, blind to the study, using a light microscope.
3.8. Immunohistochemistry Assay
IHC technique was performed using specific kits according to the manufacturer’s instructions. After deparaffinization and rehydration, endogenous peroxidase activity was blocked by 0.3% H2O2 in methanol, incubated at 4°C overnight with primary antibodies (anti-caspase 3 rabbit polyclonal antibody, 1:100 in PBS, v/v, Abcam, lat: GR224831-2). After incubation with secondary antibody, it was conjugated with horseradish peroxidase (mouse and rabbit specific HRP/DAB, Abcam, Lat: GR2623314-4) for 10 minutes; sections were incubated with diaminobenzidine tetrahydrochloride for five minutes (22). For the quantitative analysis, IHC photomicrographs were analyzed with MacBiophotonics ImageJ 1.41 software by densitometry method. The positive staining intensity was assessed as the ratio of the stained area to the entire field.
3.9. Statistical Analyses
Statistical analysis for the obtained data was performed using SPSS version 19. The results were expressed as the mean ± standard deviation (SD). One-way ANOVA and Tukey post tests were also used. P value < 0.05 was considered as the level of significance.
4. Results
4.1. Effects of ZM on Oxidative Stress
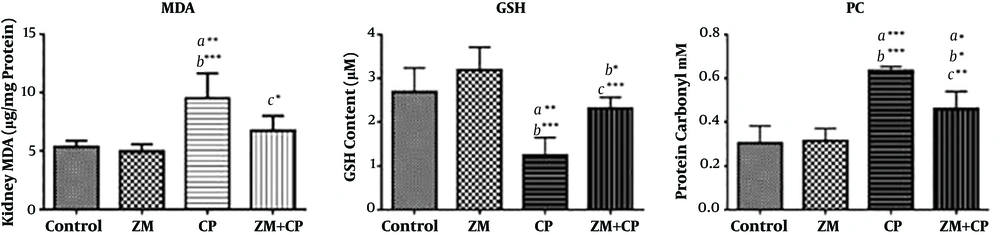
The ZM extract was standardized based on the thymol content (18, 19). The total thymol content of the extract was 9.24 ± 0.11 mg/g of dried extract. The MDA level, as the final product of lipid peroxidation, significantly increased (P = 0.001), and GSH content as an endogenous antioxidant, decreased in the CP-treated group (P ≤ 0.001) compared with the control group. In addition, PC increased in the CP-treated mice (P < 0.0001) compared with the control group. In contrast, ZM pretreatment in CP-treated mice significantly decreased the MDA and PC levels (respectively P ≤ 0.02 and P ≤ 0.009) compared with the CP group, whereas the GSH content significantly increased (P ≤ 0.003) (Figure 1).
4.2. Effect of ZM on Creatinine and Urea
CP treatment significantly increased creatinine and BUN levels. In contrast, ZM administration in CP-treated mice significantly attenuated the creatinine and BUN levels compared with those of the CP group (P < 0.05) (Table 1).
| Group | Urea, mg/dL | Creatinine, mg/dL |
|---|---|---|
| Control | 27.8 ± 0.84 | 0.28 ± 0.05 |
| ZM | 28 ± 2 | 0.33 ± 0.05 |
| DZN | 38.2 ± 2.39A***,B*** | 0.53 ± 0.1A***,B** |
| ZM + DZN | 32.4 ± 2.4A*,B*,C** | 0.38 ± 0.05C* |
Abbreviation: ZM, Zataria multiflora Boiss.
aValues are expressed as mean ± SD.
bA, versus the control group; B, versus the ZM group; and C, versus the CP group.
c*P < 0.05, **P < 0.01, and ***P < 0.001.
4.3. Histopathological Findings
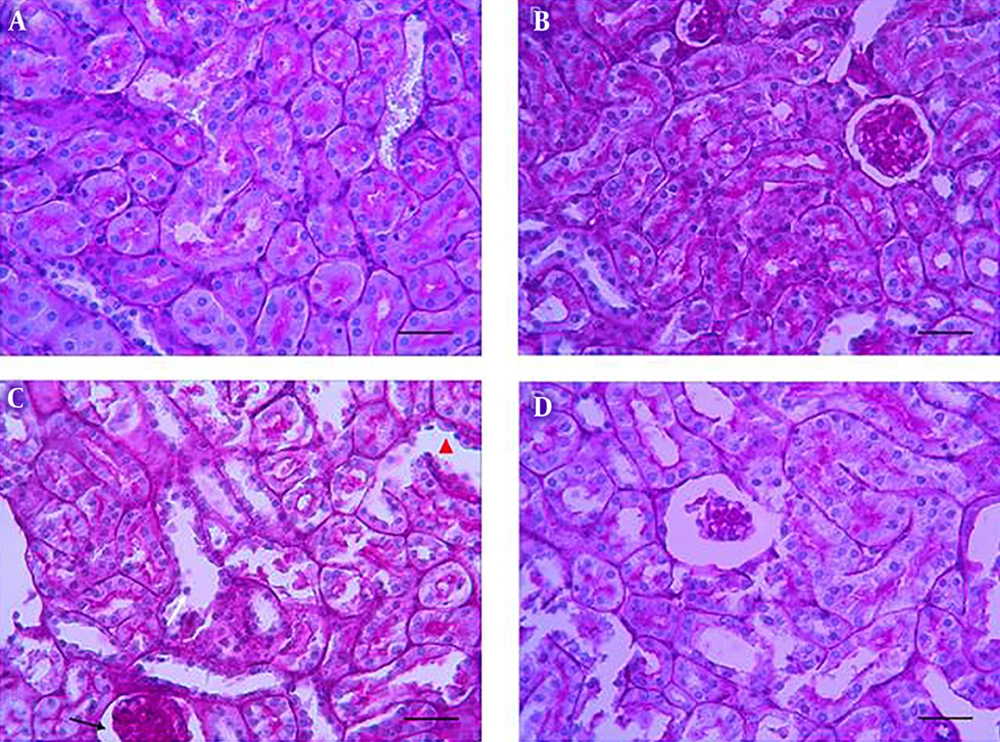
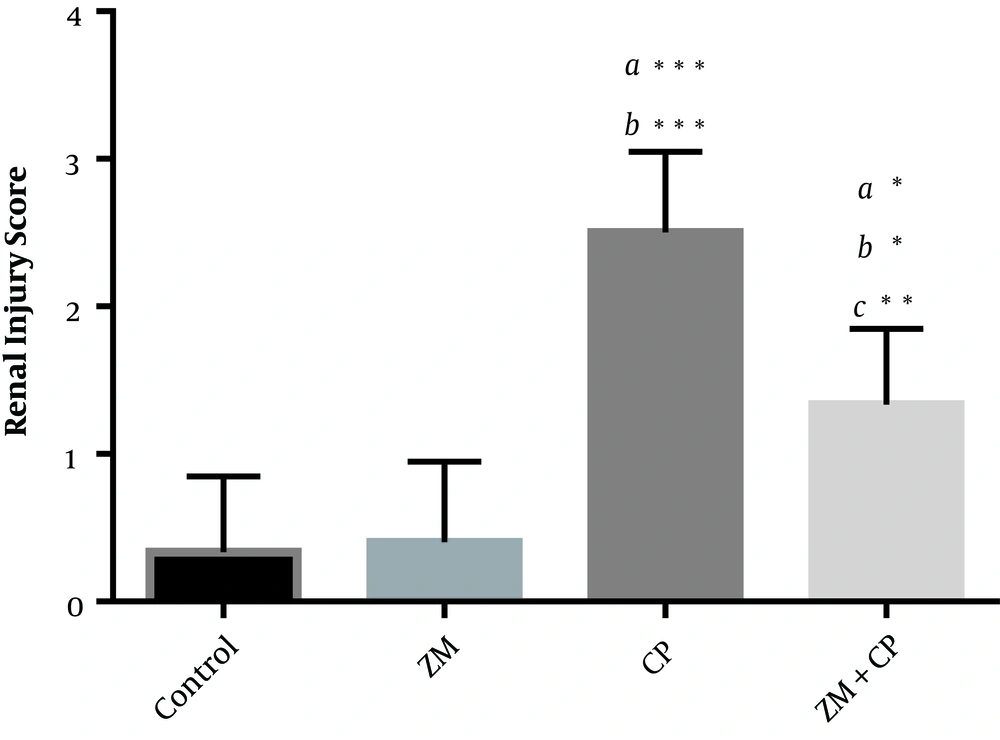
Glomerular and tubular structure of kidneys in the control group (Figure 2A) was similar to that of the ZM group (Figure 2B). CP treatment induced degeneration of epithelial cells, infiltration of leukocytes, atrophy of tubules and glomeruli, widening of Bowman’s space, pyknosis of renal epithelium nuclei, and penetration of protein in the renal proximal tubules (Figure 2C). These findings were improved in the CP + ZM group (Figure 2D). ZM therapy also ameliorated these histopathological alternations. Renal injury, as shown in Figure 3, in the CP group had a higher mean score than other groups. The injury score of the CP group was 2.5 ± 0.55, while it was 1.33 ± 0.52 in the CP + ZM group; the difference between groups was statistically significant (P < 0.006).
Photomicrographs show the effects of ZM, CP, and CP + ZM on the histological architecture of kidneys. The control (A), the ZM (B), the CP (C) and the CP + ZM (D) groups. Normal structure in the control group (A), renal tubules with wider lumen (head arrow), glomerular atrophy, dilatations of the bowman capsule (black arrow), detachment (white arrow), pyknotic nuclei of renal epithelium were observed in the CP group Mild disorganization, degeneration, edema and congestion (D) were observed in the CP + ZM group, and ZM improved these changes. (PAS staining, mag. ×400), scale bar = 100 µm.
Tubular injury was scored in renal tissue. Data are expressed as mean ± SD. The highest score belonged to the CP group. ZM decreased renal injury score in the ZM + CP group. a, versus the control group; b, versus the ZM group; and c, the versus CP group. *P < 0.05; **P < 0.01; ***P < 0.001.
4.4. Immunohistochemical Findings
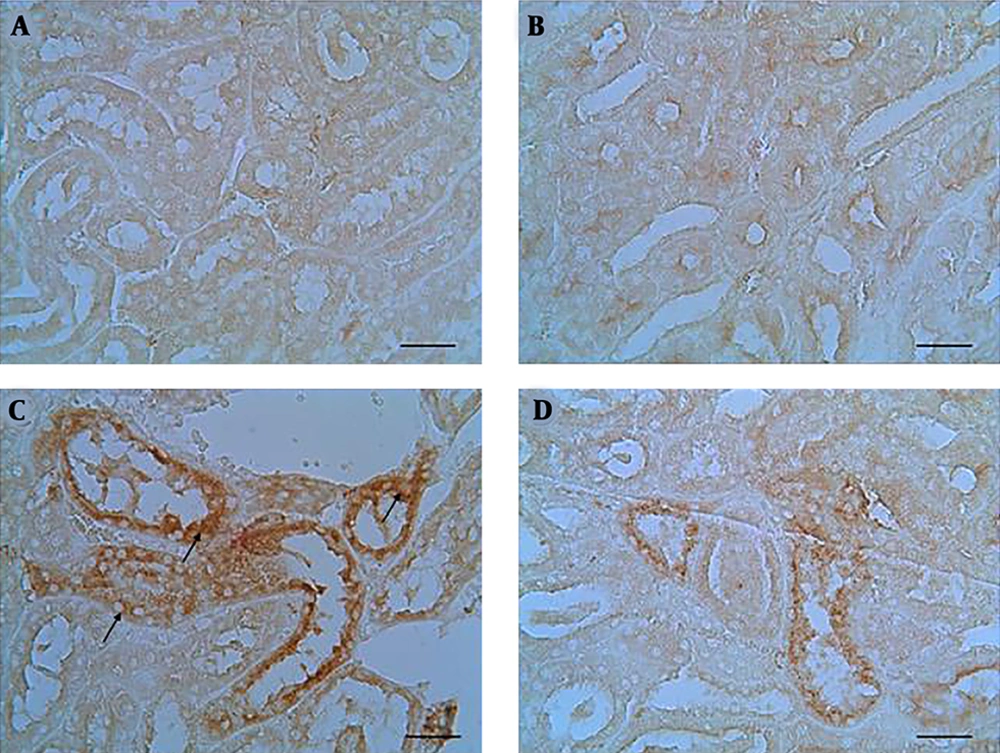
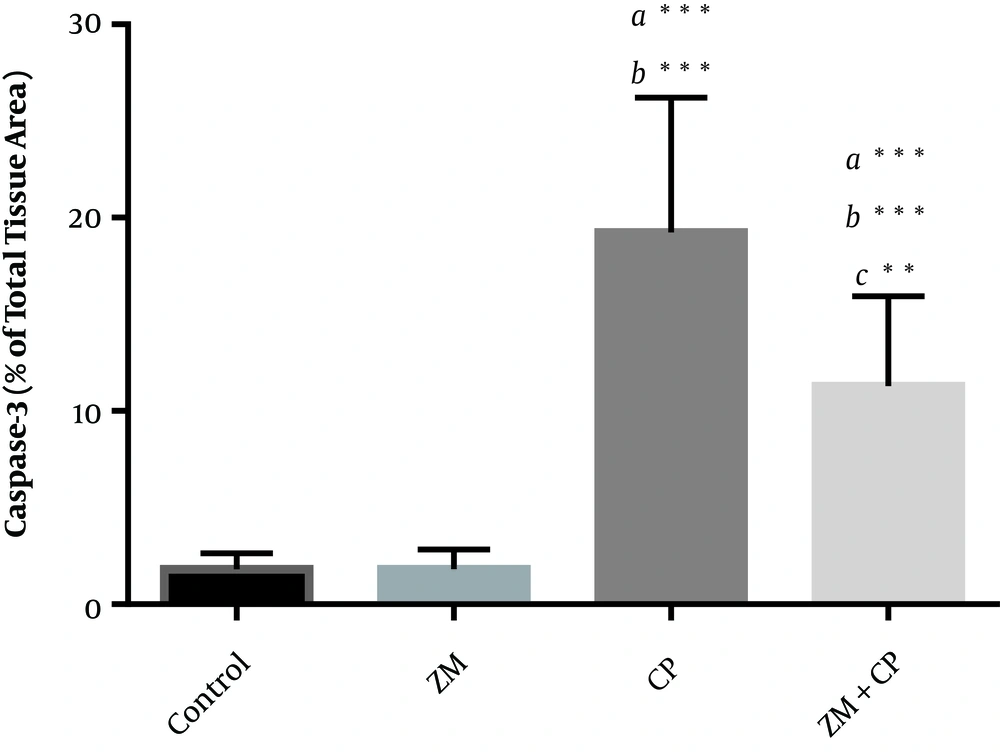
Caspase-3 positive cells were colored brown in staining. Section of kidney in the control and ZM groups showed no caspase-3 immunoreactivity. Immunoreactivity of caspase-3 was severe to moderate in the tubular epithelial cells of the cortex or medulla of kidney. Immunoreactivity of caspase-3 in the epithelial cells was assessed using staining technique and the results indicated mild immunoreactivity in the ZM + CP group compared with the CP group (Figure 4). The histograms of the semi-quantitative analysis of caspase-3 detection using IHC technique in the study groups are shown in Figure 5. The most intense immunoreactivity of caspase-3 confirmed by semi-quantitative analysis was observed in CP-treated mice (19.23 ± 6.97) compared with the other groups. ZM therapy decreased the intensity of immunoreactivity of caspase-3 (11.28 ± 4.66). Immunoreactivity level of caspase-3 in the control group was similar to that of the ZM group.
In the control and ZM groups, positive cells were not detected (A and B). Immunohistochemical staining demonstrated the caspase-3 immunoreactivity in the CP group that was remarkable in tubular epithelial cells (black arrows) (C). ZM therapy diminished caspase-3 immunoreactivity in CP-treated mice (D). (mag. ×400), scale bar = 100 µm.
Densitometry analysis of immunohistochemical staining for caspase-3; data are expressed as a percentage of total tissue area. Immunoreactivity level of caspase-3 in the control and ZM groups was similar. Data are presented as mean ± SD. a, versus the control group; b, versus the ZM group; and c, versus the CP group. *P < 0.05, **P < 0.01, ***P < 0.001.
5. Discussion
Nephrotoxicity is one of the side effects of chemotherapy drugs in patients with cancer (1). In the current study, administration of a single dose of CP (10 mg/kg) induced oxidative stress and a typical pattern of nephrotoxicity, such as a significant increase in creatinine and BUN levels. Also, CP increased immunoreactivity of caspase-3. However, ZM therapy in CP-treated mice improved oxidative stress parameters, renal failure indices, and apoptosis.
In CP therapy, increased generation of ROS induces toxicity (2). ROS directly affects proteins, lipids, and nucleic acids of cells and induces cellular injury and necrosis in the different tissue (23). The current study findings showed that CP significantly increased MDA as lipid peroxidation marker and decreased GSH that confirmed induction of oxidative stress. Some studies reported increased MDA levels due to CP-induced nephrotoxicity (3). GSH, as the most important intracellular antioxidant, plays an important role in the repair of damages caused by oxidative stress (24). Also, CP, by increasing the ROS, induces the oxidation of proteins, and subsequently, increases the carbonyl protein levels (25). In the current study, CP therapy increased MDA and PC content and decreased GSH levels in the kidney; these findings were in line with those of other studies (25, 26). Oxidative stress is produced by the imbalance between pro-oxidant and antioxidant chemical species. Therefore, antioxidant administration can reduce oxidative damage, which delays the progression of kidney injury. ZM, due to its antioxidant properties, alleviated oxidative stress and lipid peroxidation in the current study. Antioxidant enzymes are the first line of defense against ROS. HPLC analysis showed that ZM contains thymol and carvacrol as phenolic compounds (18, 19). Some other studies also showed that these compounds, with scavenging free radicals can protect tissue against oxidative stress and improve injury by increasing GSH and reducing lipid peroxidation (27). The results of the current study suggested that ZM by maintaining antioxidant capacity may have nephroprotective activity. In CP therapy, necrosis and epithelial cell injury mainly localize to S3 subsegment of proximal tubules (28). Other studies demonstrated protective effects of ZM against nephrotoxicity induced by gentamicin (29). Also, ZM could protect liver against CP (30). In the present study, ZM supplementation in CP-treated mice greatly decreased histopathological alterations and significantly improved the histoarchitecture of renal tubules that was approximately close to the findings in the control group. Creatinine and BUN are the indicators of kidney dysfunction (2). In the present study, ZM mitigated tubulointerstitial injuries and renal dysfunction in CP-treated mice. Also, ZM recovered renal function close to normal levels when co-administered with CP.
CP is a simple inorganic molecule that binds to DNA and leads to inter- and intra-strand cross-links formation. Cross-linking of DNA induces DNA damage that can lead to irreversible injury and cell death (31). On the other hand, ROS is an important factor that induces apoptosis by the activation of caspases pathways. Caspase-3 as an essential apoptotic effector, through affecting cytoskeletal and nuclear chromatin, induces apoptosis (32). Therefore, anti-apoptotic agents are capable of minimizing apoptosis in nephrotoxicity induced by CP (33). In the current study, results of IHC assay and caspase-3 positive cells indicated a significant DNA damage induced by CP in the renal epithelial cells. Caspase-3 activation was induced eight hours after CP treatment and its amount increased by 54 times after 24 hours (34). The current study also demonstrated that treatment with ZM in CP-treated mice significantly decreased apoptosis in kidney. A study showed that thymol can significantly attenuate CP-induced nephrotoxicity (17). Also, thymol and carvacrol, as bioactive components of ZM, can decrease oxidative stress, inflammation, and apoptosis. They also proved that these components have protective effect against nephrotoxicity induced by CP (27).
In summary, the renal cell injury induced by CP. In addition, the renal dysfunction factors in CP-treated mice were markedly alleviated by ZM extract. The nephroprotective effect of ZM was associated with the reduction of oxidative stress and apoptosis. These findings proved that ZM has a nephroprotective effect via anti-oxidant and anti-apoptotic properties.