1. Background
Anxiety disorders are a class of mental and behavioral disorders with a high prevalence among the general population. Anxiety disorders markedly impact the function and quality of life (1). More than one-fifth of the population will suffer from anxiety in their lifetime (2). According to the World Health Organization, depression is speculated to be the second most common cause of the premature disability and death by 2020 (3).
Currently, pharmacotherapy is considered a routine and efficacious treatment for anxiety disorders (4). Sedative-hypnotic medicines are usually administered to control these disorders; among them, benzodiazepines are one of the most common types. However, their use has been restricted by the severe adverse effects, including anterograde amnesia (5). Withdrawal symptoms, sedation, and dependence occur with long-term administration of benzodiazepines (6). According to these adverse effects, many pharmaceutical companies and researchers have decided to introduce complementary or herbal medications with specific anxiolytic effects and fewer side effects.
At the present time, herbal therapy is proposed as an effective alternative approach for the treatment of certain neurological disorders. Many studies have been conducted on different animal models to demonstrate the beneficial effects of plant-derived medications (7). Phytoestrogens are plant constituents with a similar structure and function to estrogens, including estradiols, which can bind to estrogen receptors (ERs), selectively (8). Some of the animal studies have indicated the beneficial role of soya as a phytoestrogen in the amelioration of the anxiety symptoms (9, 10).
Vitex agnus-castus, also known as a chaste tree (11), is endemic to Central Asia and Mediterranean Europe and belongs to the family Verbenaceae (12). It is a source of phytoestrogens and contains flavonoids and apigenins (13). It has been used traditionally to treat some conditions in females, including premenstrual syndrome, menstrual disorders (dysmenorrheal and amenorrhea), acne, infertility, corpus luteum insufficiency, hyperprolactinemia, lactation problems, and menopause (14-16).
Previous studies have indicated that V. agnus-castus has a modulatory effect on anxiety through interactions with neurotransmitters and neural system, such as dopaminergic and serotonergic neurotransmitters (17, 18). In contrast, phytoestrogens from V. agnus-castus have a high affinity for ER binding, as well as stimulation of progesterone receptor expression (19).
Incompatible findings have been reported regarding the association of mood changes with the level of hormones. Notable findings with respect to V. agnus-castus are described below:
1. Estrogen promotes opiate-containing neuron activities and enhances β-endorphin synthesis and release (20).
2. Estrogen through direct modulation of dopaminergic activity promotes releasing dopamine in the hypothalamus and improves dopamine transmission, as well as D2 receptors (21).
3. Estrogen, and consequently phytoestrogen, may alter GABA receptors; these messengers may affect GABA activity (22-24).
2. Objectives
Since estrogens contribute to anxiety and phytoestrogens imitate estrogens, in this study, the effects of phytoestrogens from V. agnus-castus on anxiety were examined in female rats, using the elevated plus-maze (EPM) test; furthermore, we attempted to determine the possible involvement of the estrogenic system.
3. Methods
3.1. Animals
For the experiments, female Swiss mice (body weight: 18 - 22 g) were maintained under standard laboratory conditions in the animal house of School of Pharmacy, Ahvaz University of Medical Sciences (Ahvaz, Iran). Eight rats were kept in cages at 23 ± 1°C in a 12:12 hours light-dark cycle. The animals had free access to food and water and were given time to adapt to the conditions (1 week) before the test. The animals were euthanized immediately after each experiment. Guidelines for animal care of Ahvaz University of Medical Sciences were respected during the study.
The mice were classified into seven groups (eight per group), and the treatment groups were administered by different doses of V. agnus-castus extract (25, 50, and 100 mg/kg). Over four consecutive days, treatments were administered intraperitoneally (i.p.). The EPM test was applied at 30 minutes following the final administration. The positive and negative control groups received diazepam (0.5 mg/kg) and normal saline (1 mL/kg), respectively. The tamoxifen group received 15 mg/kg of tamoxifen. The final group received V. agnus-castus extract (100 mg/kg) and tamoxifen.
3.2. Preparation of Methanol Extract
The methanol extract of V. agnus-castus fruit was purchased from Pursina Company (Tehran, Iran). Alcohol was evaporated at 35°C and the dry powder of V. agnus-castus was obtained.
3.3. EPM Test
The EPM test is a valid method to screen and evaluate antianxiety effects in the rodents (25, 26). The maze includes 2 open and 2 closed arms (30 × 5 × 25 cm), which expand from a common platform (5 × 5 cm). The wooden maze was elevated to 45 cm above the floor, with a 0.25 cm edge on the perimeter of the open arms. The experimental groups were administered 25, 50, and 100 mg/kg of V. agnus-castus extract for 4 consecutive days. The positive and negative control groups received diazepam (0.5 mg/kg) and normal saline (1 mL/kg), respectively.
The tamoxifen group received 15 mg/kg of tamoxifen. After 30 minutes, the final group received V. agnus-castus extract (100 mg/kg) following tamoxifen injection (15 mg/kg). Each group was placed at the center of the maze at 30 minutes post injection, while facing an open arm. The frequency of entries to the open and closed arms, as well as time spent in the arms, was recorded during the 5-minute test (26).
The anxiolytic effect of various treatments was determined based on the spent time in the open arms. To develop an activity index, entrance into each arm, besides total entry time, was measured. The placement of all 4 paws onto the arm was considered to represent entrance. After each test, ethanol solution was used to clean the maze.
3.4. Statistical Analysis
For data analysis, SPSS version 13 was used, and ANOVA and post-hoc Tukey test were performed. The significance level was set at 0.05. Values are expressed as mean ± SEM.
4. Results
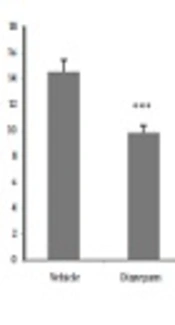
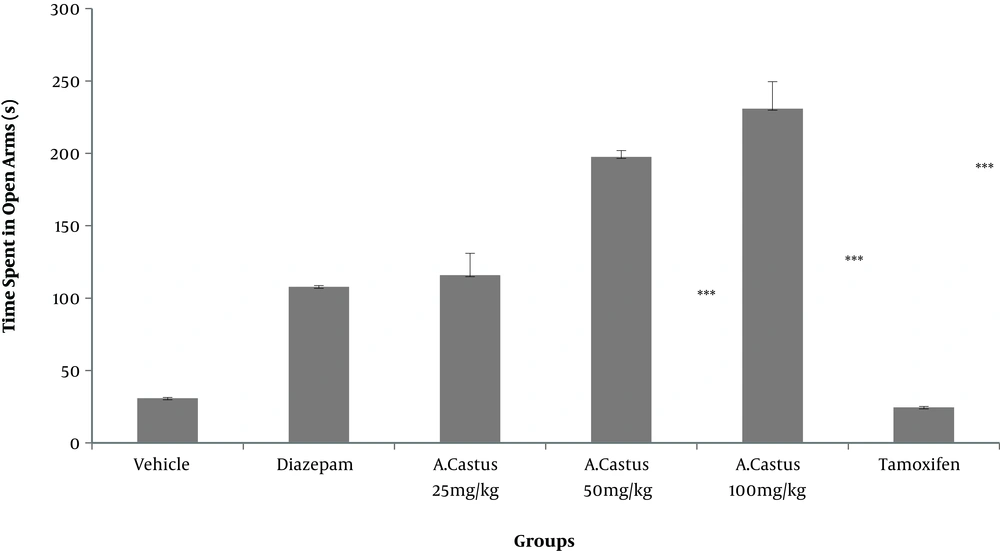
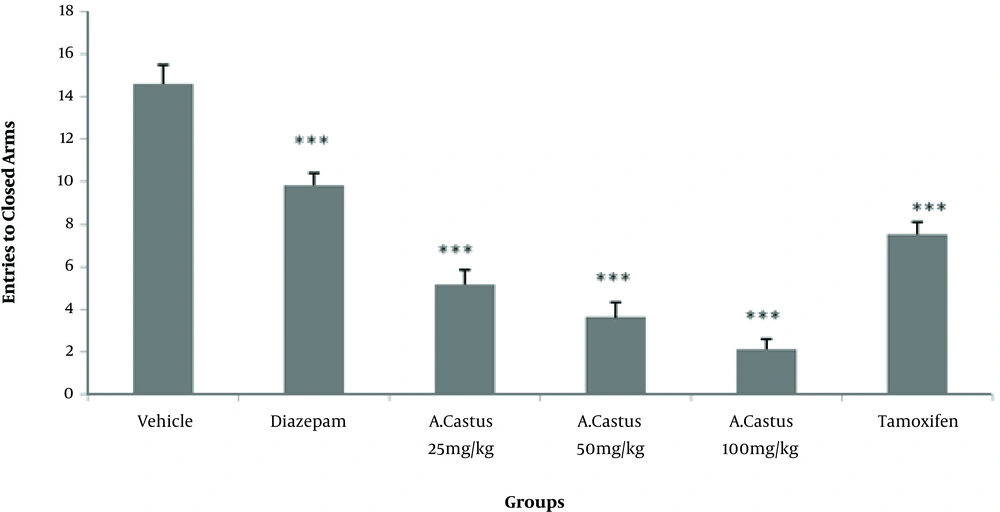
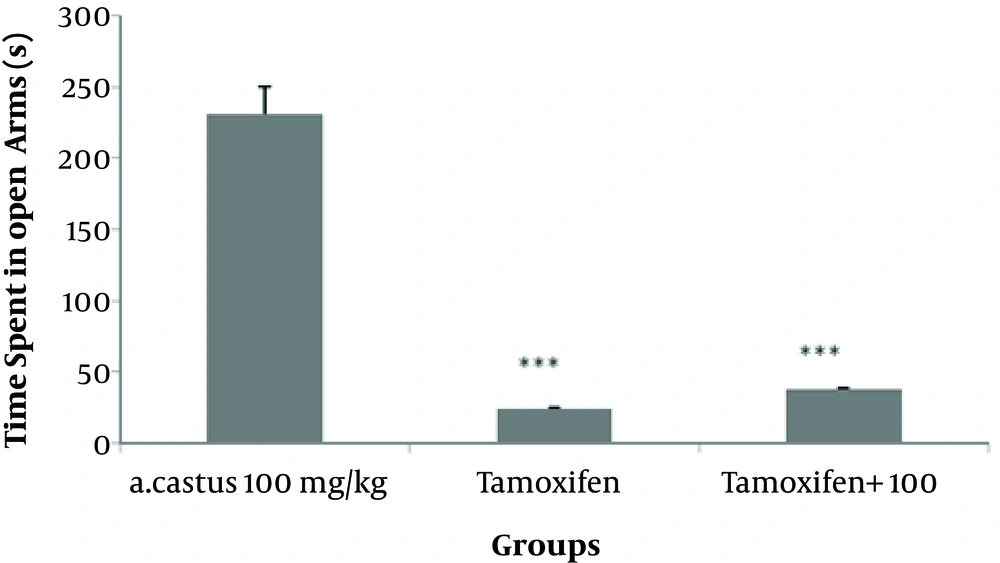
According to the results, more time was spent in the open arms in mice receiving different doses of V. agnus-castus extract and diazepam than the saline group (P < 0.001). However, the difference was not significant between the tamoxifen and saline-treated groups (Figure 1). As shown in Figure 2, the groups treated with different doses of V. agnus-castus extract, tamoxifen, and diazepam experienced a significant decrease in the frequency of entries to closed arms than the saline group (P < 0.001). As shown in Figure 3, the spent time in the open arms significantly reduced in groups receiving tamoxifen and tamoxifen plus 100 mg/kg V. agnus-castus, compared with the group treated with 100 mg/kg of V. agnus-castus alone.
The effects of V. agnus-castus (100 mg/kg) extract, tamoxifen, and a combination of tamoxifen and V. agnus-castus extract (100 mg/kg) on the spent time in the open arms during the 5-minute test. Data are presented as mean ± SEM.***P < 0.001 indicates significant differences compared with the high-dose V. agnus-castus extract group (100 mg/kg).
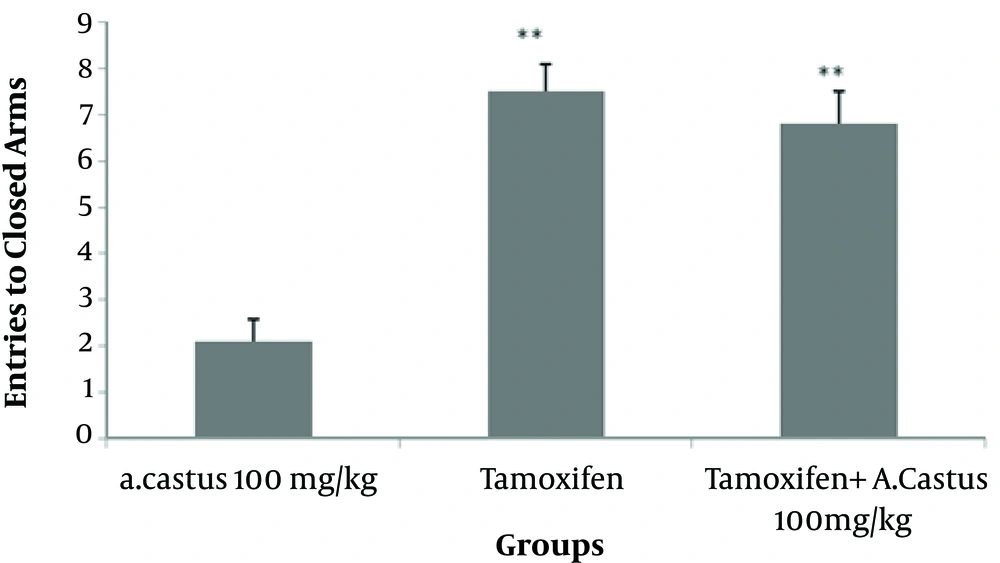
The results also showed that the number of entries to the closed arms significantly increased (P < 0.001) in tamoxifen and tamoxifen plus 100 mg/kg V. agnus-castus groups, compared with the group treated with 100 mg/kg extract (Figure 4).
Effects of V. agnus-castus extract (100 mg/kg), tamoxifen, and combination of tamoxifen and V. agnus-castus extract (100 mg/kg) on the frequency of entries to the closed arms on the 5-minute test. Values are presented as mean ± SEM. **P < 0.01 in comparison with the high-dose V. agnus-castus extract (100 mg/kg).
5. Discussion
To examine the anti-anxiety effects of hydroalcoholic V. agnus-castus extract, an EPM model of anxiety was applied. The model focuses on the rats' natural reticence to avoid open and elevated places (27). First, we examined the antianxiety activity of different doses of V. agnus-castus extract, as well as the effect of tamoxifen on anxiety. Finally, we administered tamoxifen to block ERs and then applied an effective dose of V. agnus-castus extract to assess the possible role of ERs. According to the findings, different doses of the extract (including 25, 50, and 100 mg/kg) were effective.
In this study, tamoxifen, which is an effective nonselective ER antagonist, was used (28). Administration of tamoxifen to mice blocks ERs. Therefore, tamoxifen induces anxiety-like behaviors in animals. A high dose of V. agnus-castus extract (100 mg/kg) reduced the anxiety symptoms in the animals, whereas coadministration of tamoxifen and high-dose V. agnus-castus extract reversed the anti-anxiety effects of V. agnus-castus extract and caused the mice to exhibit anxiety. Inhibition of the anti-anxiety effects of V. agnus-castus extract may be related to the blockade of ERs induced by tamoxifen.
In the different pharmacological studies, diazepam has been used as a reference sedative-hypnotic drug. Our findings revealed the substantial anti-anxiety effects in animals receiving diazepam. Loch et al. indicated that V. agnus-castus caused a decrease in depression and anxiety disorders in females with premenstrual syndrome (PMS) (29). Schellenberg et al. evaluated the impact of V. agnus-castus on other PMS symptoms, such as anxiety, headache, mood swings, agitation, and breast pain. Their findings showed its beneficial effects on the alleviation of symptoms (30).
The fruits of V. agnus-castus contain iridoids and flavonoids; in addition, the leaves and flowers contain compounds that are structurally similar to sex hormones (31). The available knowledge suggests that phytoestrogens are suitable for the prevention and treatment of several diseases (32). Phytoestrogens are the analogs of estrogens and have estrogenomimetic properties with a high affinity for binding to ERs; however, their ability is weaker than endogenous estrogens (33). They originate from vegetables and have a high therapeutic potential (32).
In the present study, V. agnus-castus was used for its phytoestrogen content. Studies have previously indicated the anxiolytic effects of phytoestrogens, such as, soya and fennel (9, 34). Two types of ERs are present, ER-α and ER-β. According to studies on rats and mice, ER-β is responsible for the anxiolytic effects of estrogens (35, 36). To elucidate the role of ER-α and ER-β in anxiety, it has been demonstrated that the anxiety behavior increased in female ER-β knockout mice, whereas no differences were seen in ER-α knockout mice (37).
ER-β is more expressed in the amygdala and paraventricular nucleus of the hypothalamus, which are related to fear and the anxiety responses (38, 39). Hence, the inability of estradiol to act on these brain areas may explain the anxiety symptoms in ER-β knockout mice (37). However, since phytoestrogens possess estrogenic actions (40) and have a greater affinity to ER-β (8), these receptors may contribute to the anxiolytic-like effects of V. agnus-castus.
Dietary phytoestrogens, such as, genistein have a high affinity and activity for ER-β and have been shown to decrease anxiety in both female and male rats in EPM test (39). V. agnus-castus extract exerted anti-anxiety effects through interference in the dopaminergic and serotonergic systems (17, 18). In this study, the importance of ERs in anxiety was investigated, and the anti-anxiety potential of V. agnus-castus may be associated with estrogen system modulation.
These findings were partly consistent with previous research, demonstrating the anxiolytic effects of selective ER-β modulators in the open field (17 β-estradiol, diarylpropionitrile, and 7, 12-dihydrocoumes), EPM test, light-dark transition, emergence, Vogel punished drinking tasks, and defensive freezing, inhibited via tamoxifen coadministration (41).
Consumption of phytoestrogen influences the fertility and morphogenesis of ovaries in animals (42). As phytoestrogens may change the level of sex hormones in consumers, the treatment doses of phytoestrogen should be precisely determined (43). Eventually, the efficiency of isoflavones in animal and in vitro studies and clinical trials should be evaluated to confirm their beneficial effects on humans.
5.1. Conclusions
Our study results indicated that V. agnus-castus extract exerted anxiolytic effects in mice subjected to the EPM test. When ERs were blocked by the administration of tamoxifen, the inhibitory effects of the extract on anxiety diminished. The present study supports previous research, reporting the effectiveness of phytoestrogens in the reduction of anxiety. Also, ERs may be responsible for the antianxiety effects.