1. Background
Cutaneous leishmaniasis (CL) is an important zoonotic disease worldwide (1, 2). Currently, pentavalent antimonials, such as meglumine antimoniate (Glucantime®), are used as first-line treatment for CL (3). However, these medicines pose some health risks due to their inherent toxicity (4), especially when systemic therapy is necessary for CL (5).
In recent years, many reports have been published regarding the increasing frequency of drug resistance in Leishmania species to antimonial compounds (6). Accordingly, many researchers have tried to develop new agents for the treatment of CL (7). One of the major approaches to the treatment of various types of leishmaniasis is the use of medicinal plants (8). The present study aimed to examine the effects of various fractions of Falcaria vulgaris on Leishmania major.
The genus Falcaria, which belongs to the family Apiaceae, is an herbaceous plant with 2 species endemic to Iran, i.e., F. vulgaris and F. falcarides. Spathulenol and carvacrol are 2 essential oils found at high concentrations in F. vulgaris extracts. Additionally, germacrene B is the most frequently found essential oil in F. falcarioides extracts (9). A previous study revealed that some essential oil compounds of this plant have antileishmanial activities (10).
The use of F. vulgaris in the treatment of skin wounds dates back to several decades ago. In a study conducted by Khazaei and Salehi, the effects of F. vulgaris extract on gastric ulcer were evaluated in animal models; the results showed that the ethanolic extract could significantly reduce the ulcer index (11). Likewise, another study confirmed the valuable effect of this plant on gastric ulcers (12). The therapeutic effects of F. vulgaris on skin wounds have been also discussed in the literature (13). In this regard, a previous study confirmed the antibacterial effects of this plant (14). However, no study has yet examined the effects of F. vulgaris extract on L. major, as the causative agent of skin wounds.
2. Objectives
We aimed to evaluate the in vitro antileishmanial activities of aqueous, ethyl acetate, and butanol fractions of F. vulgaris on L. major promastigotes.
3. Methods
3.1. Parasites
The standard strain of L. major (MRHO/IR/75/ER) was provided by the department of parasitology, Isfahan University of Medical Sciences, Isfahan, Iran. The parasites were maintained under laboratory conditions, allowing the repeated passage of BALB/c mice. Amastigotes were isolated from the infected mice and transformed into promastigotes in Novy-MacNeal-Nicolle (NNN) medium. The promastigotes, obtained from the third passage in the NNN medium, were gradually added to RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS), 100 µg/mL of streptomycin, and 100 IU/mL of penicillin at 25°C.
3.2. Plants
The aerial parts of F. vulgaris were collected in the spring from Khomein, Markazi Province, Iran. Subsequently, the collected plants were authenticated at the department of pharmacognosy, Isfahan University of Medical Sciences, Isfahan, Iran and stored in the herbarium (No., 2955).
3.3. Total Extract
The collected plants were left in darkness at room temperature to dry and were then ground to pass through a sieve. The powder was extracted using the percolation method (15), and the total extract was concentrated by a rotary evaporator.
3.4. Chlorophyll and Oil Elimination
Chlorophyll and oil were eliminated from the total extract via medium pressure liquid chromatography (MPLC), using petroleum ether and acetone (16).
3.5. Total Extract Fractionation
Fractionation was performed using the liquid-liquid extraction (LLE) method (17). Equal volumes of ethyl acetate and distilled water were mixed. The total extract was added to a separatory funnel, stirred vigorously, and allowed to form 2 separate phases. Subsequently, ethyl acetate was separated from the aqueous phase. Afterwards, butanol was added to the remaining aqueous phase at the same volume as the aqueous phase, stirred vigorously, and allowed to form 2 phases; then, the butanol phase was separated from the aqueous phase; the remaining aqueous phase was also collected. The fractions were lyophilized and kept at -20°C until further use.
3.6. Experimentation
Initially, butanol and ethyl acetate fractions were dissolved in isotonic saline, containing dimethyl sulfoxide (DMSO); it should be noted that the final volume of DMSO in the testing medium was approximately 0.2%. DMSO was not added to the aqueous fraction. Each fraction was studied at 6 concentrations (40, 80, 160, 320, 640, and 1280 µg/mL) in the RPMI medium, supplemented with FBS (10%), 100 µg/mL of streptomycin, 100 IU/mL of penicillin, and 1 × 106 exponentially growing promastigotes.
The single-blind experiments were performed in triplicate for each concentration at each interval (6, 24, 48, and 72 hours). It should be noted that a positive control containing amphotericin B (1 µg/mL) and the parasite, as well as 2 negative controls (1 with 0.2% DMSO and parasite and 1 with only the medium and parasite), was considered each time.
The negative control with DMSO was used for the ethyl acetate and butanol fractions, while the control without DMSO was applied for the aqueous fraction. At each interval (6, 24, 48, and 72 hours), 10 µL of each sample was gently mixed with 10 µL of 0.4% trypan blue on a glass slide. The mortality of parasites was determined by counting the number of alive (not stained) and dead (stained) promastigotes according to the following formula (1).

Accordingly, at least 50 microscopic fields were examined for each sample.
3.7. Data Analysis
The normal distribution of data was evaluated using nonparametric Kolmogorov-Smirnov test. Interactions between concentration and time were separately analyzed by repeated measures analysis of variance (ANOVA) for each fraction. The groups were compared using one-way ANOVA test, followed by Tukey’s honest significant difference (HSD) test at P value below 0.05. The Microsoft Excel software (Microsoft Inc.) was used to determine the lethal dose 50 (LD50) by plotting the percentage of death versus different concentrations of each fraction at a given time.
Considering the nonlinearity of the effect of each fraction at different concentrations, type II regression analysis was performed; by putting Y = 50 in the equation, X values were calculated. The half-maximal inhibitory concentration (IC50) was calculated for only the butanol fraction at a concentration of 1280 µg/mL within 48- and 72-hour intervals, which produced the greatest effects, respectively. The statistical analyses were performed using SPSS version 16.
4. Results
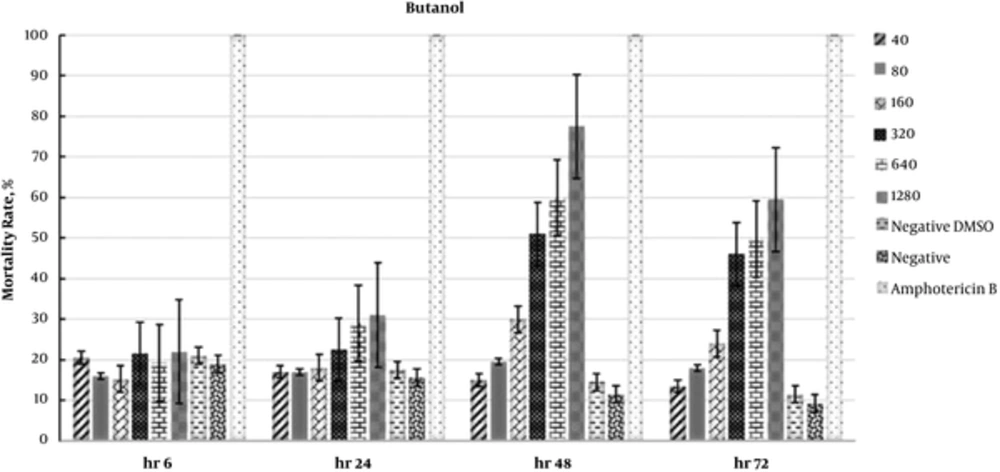
The results of repeated measures ANOVA showed no significant differences for the aqueous (P = 0.16) and ethyl acetate (P = 0.22) fractions, whereas a significant difference was observed for the butanol fraction (P = 0.04). One-way ANOVA revealed a significant difference among fractions at 48 and 72 hours (P = 0.04 for both). Moreover, Tukey’s HSD test showed a significant difference between the butanol fraction and the other 2 fractions (P < 0.05) at 48 and 72 hours.
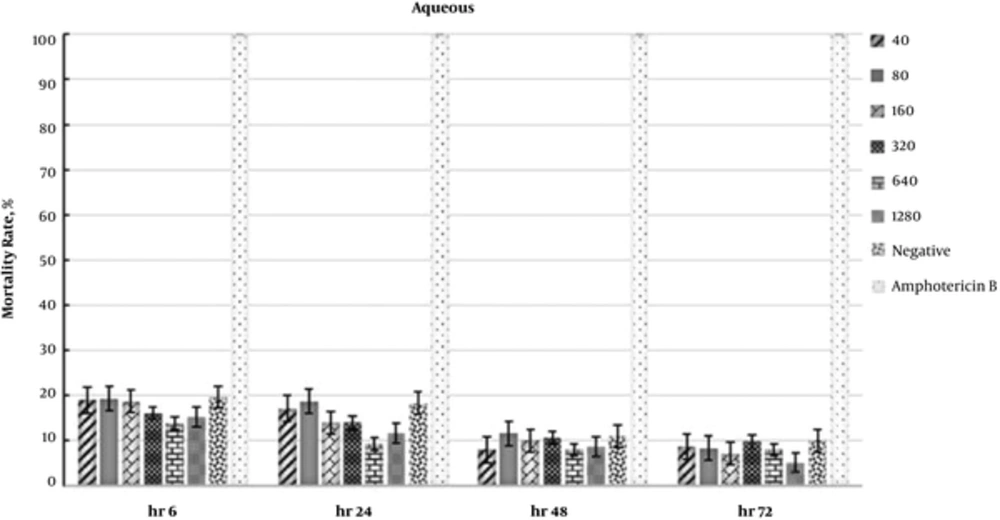
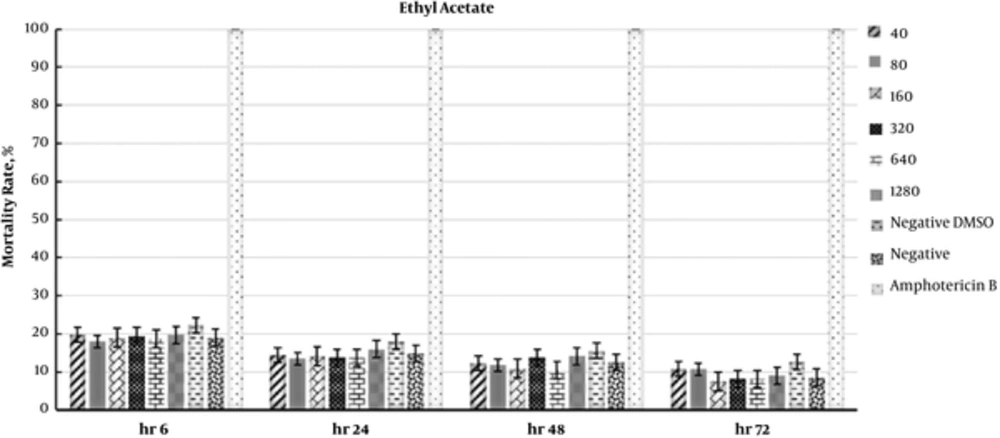
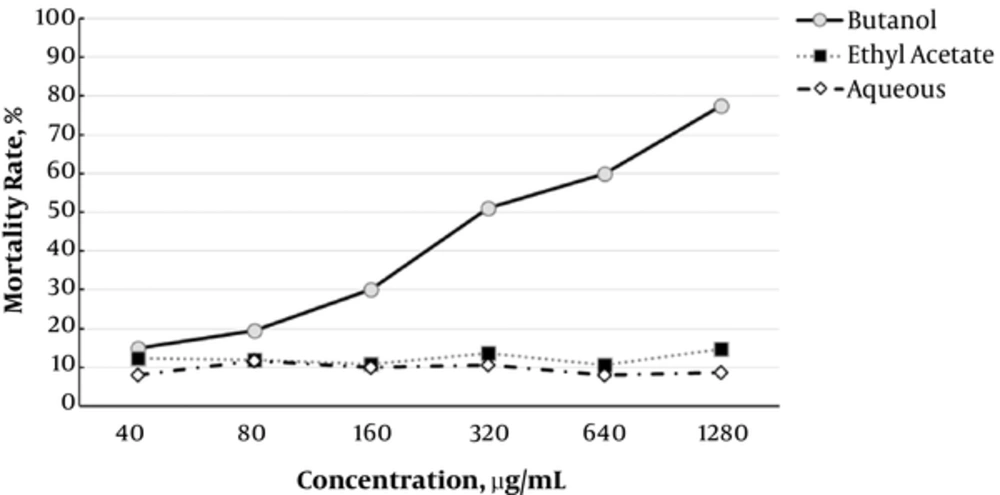
In addition, the IC50 values for the butanol fraction were 343.8 and 639.1 µg/mL at 48 and 72 hours, respectively. The data regarding the antileishmanial effects of fractions are presented in Figures 1 - 3. Moreover, the effects of F. vulgaris fractions on L. major promastigotes at 48 hours are compared in Figure 4. The butanol extract showed maximum antileishmanial effects in comparison with other fractions.
5. Discussion
Considering its efficacy in the treatment of wounds, F. vulgaris was examined in the present study (18). Previous studies have shown that beta-caryophyllene, alpha-pinene, and limonene are compounds with antileishmanial activities (19-21). In this regard, one previous study showed that F. vulgaris is rich in these compounds (10). In addition, saponin is another compound in this plant with antileishmanial effects (22, 23).
According to some research, a few plants show mild to strong activities against Leishmania species (24-27). Asadi et al. (28) showed that the hydroalcoholic extracts of Stachys lavandulifolia and Mespilus germanica have antileishmanial activities. Some studies have also shown that these extracts are suitable adjuvants in vaccination against Leishmania species (29, 30). Recently, invivo studies have revealed that alcoholic extract of Hedera helix and ethanol extract of Peganum harmala exert antileishmanial effects on L. major (31, 32). Furthermore, another study showed that the aqueous extract of Artemisia sieberi has more antileishmanial activities than artemisinin (33).
Moreover, the mucilage extract of Cordia myxa has shown antileishmanial activities against L. major and L. infantum (34). The results of a previous study on the effects of 6 plants showed that Allium hirtifolium and Ziziphus spina-christi had greater inhibitory effects on parasites (35). Another study showed that the extracts of Stachys lavandulifolia Vahl. and Mespilus germanica L. leaves could inhibit the proliferation of L. major promastigotes.
In the present study, the aqueous fraction of F. vulgaris showed the greatest effect on parasite death during the first 6 hours (Figure 1). After this interval, changes reduced at all concentrations, and the difference was almost insignificant among different concentrations within 48 and 72 hours. As most solute compounds in the aqueous fraction are proteins and carbohydrates, they may act as a source of energy for parasite survival and proliferation; this may be the reason for the reduced death of parasites in this interval.
Likewise, the effects of ethyl acetate fraction on parasite mortality were almost similar to the aqueous fraction. In addition, the greatest effect of ethyl acetate fraction was observed during the first 6 hours. The changes were almost similar for all the fractions during the first 24 hours. In this study, the highest antileishmanial effect was observed for the butanol fraction with an IC50 of 343.8 µg/mL at 48 hours. As presented in Figure 3, the antileishmanial effect of butanol fraction was less significant than amphotericin B; in fact, amphotericin B destroyed 100% of parasites within 6 hours. Nevertheless, the butanol fraction showed a reliable and dose-dependent antileishmanial effect at 48 hours (Figure 3).
So far, researchers have introduced various antileishmanial compounds (36). Accordingly, the world health organization (WHO) has recommended the use of medicinal plants in the treatment of leishmaniasis (34). Based on the present findings, F. vulgaris has acceptable antileishmanial activity and can act as an effective agent in the treatment of wounds without any side effects. Therefore, it may be used as a useful complementary or alternative treatment for skin lesions of cutaneous leishmaniasis. However, further invivo studies are necessary in the future.