1. Background
Herbal products are commonly categorized as food, dietary supplements, herbal medicinal products, and cosmetic materials (1). There is growing knowledge and public acceptability regarding the usage of herbal drugs in today’s medicine in both developing and developed countries. In addition to drugstores, medicinal plants and herbal products are widely available via other retail outlets, for example markets, stores, and mail order (2).
Lack of regulation, loose distribution channels, poor cultivation conditions, and a myriad of environmental factors during the post-harvest and manufacturing stages can result in poor quality of herbal products. Accordingly, the safety of herbal products and their contamination with harmful pathogens have become a major public health concern (3).
Medicinal herbs can be contaminated with a variety of bacteria and their spores, fungi, viruses, protozoa, and other organisms (2). Gastrointestinal tract of humans and animals is the dominant habitat of enterococci. Because of their high heat tolerance and survival under harmful environmental conditions, these pathogens are considered as the indicators of the hygienic quality of food. These bacteria are abundantly present in foods, vegetables, and plant materials (4), and the resulting infections are usually hard to treat due to the increased antibiotic resistance (5).
Enterococci can cause food poisoning through the production of biogenic amines and can cause opportunistic infections (4). Pathogenic microorganisms, including Enterobacter, Enterococcus, Clostridium, Pseudomonas, Shigella, and Streptococcus, have been shown to contaminate herbal products.
It goes without saying that it is necessary to curtail the risk of microbial contaminations (6), and there are quality standards that could be considered as guidelines. According to Iran Standard National Organization, the standard limit for enterococci is zero per gram (0/gr) of dehydrated herbs (7). Conventional microbiological methods have been used as standard for the detection of pathogens, but traditional microbiological methods are time-consuming and labor-intensive and are therefore, not suitable for a timely response to possible risks. As a result, many rapid methods have been developed to reduce experimentation time (8). The impedance method has been applied in the field of microbiology for the detection and quantification of bacteria. Impedance measurement is based on the evaluation of changes in electrical impedance of a culture medium during bacterial growth and metabolic processes that generate electrically measurable changes in the medium (8-10).
2. Objectives
In this study, we sought to detect enterococci contamination in industrial and traditional medicinal herbs by standard and impedance methods.
3. Methods
3.1. Media, Supplements, and Medicinal Herbs
KF Streptococcus agar (Merck, Germany), Bromocresol Purple Azide broth (Quelab, Canada), Bile Esculin Azide agar (Merck, Germany) BiMedia 330A (Kanamycin Esculine), and Azide broth (BiMedia Sy-Lab, Austria) were prepared according to the manufacturers’ instructions. Triphenyl tetrazolium chloride (TTC) was purchased from Merck Millipore. In this study, 80 samples of industrial and traditional medicinal herbs including valerian (Nardostachys jatamansi), licorice (Glycyrrhiza glabra), fennel (Foeniculum vulgare), and avishan shirazi (Zataria multiflora) were collected randomly from grocery shops, drugstores, retailers, and manufacturers of herbal medicinal products.
3.2. Standard Method
For enterococci detection, 1 g of the sample was inoculated in 10 mL of glucose broth medium (Bromocresol Purple Azide broth) for enrichment. Then, the medium was incubated at 37°C for 48 h. In case the color of the glucose culture medium changed to yellow, it was cultivated on KF Streptococcus agar containing 1% TTC. The plates were incubated at 37°C for 48 h. Suspected colonies of Enterococcus were detected as a result of TTC reduction and conversion to formazan with red, purple, pink, and chestnut colors. Then, the esculin hydrolysis test was performed on suspected colonies as a confirmatory test. For this purpose, the selected colonies were transferred to Bile Esculin Azide agar medium and incubated at 37°C for 48 h. Intestinal enterococci hydrolyze esculin and produce dark brown to black colonies (11).
3.3. Impedance Method
All the samples were also analyzed using the impedimetric monitoring technique (Bactrac 4300 microbial analyzer, Sy-Lab Company). For this purpose, 0.1 mL of Bromocresol Purple Azide broth was inoculated into impedance glass tubes (cells) containing 9.9 mL of BiMedia 330A (Kanamycin Esculin Azide broth). Simultaneous specifications of the tube samples were added via System software. The evaluation protocol was set at 37°C and 10-minute intervals for measuring impedance for 24 h. In our study, electrical resistance changes were measured every 10 minutes in BiMedia 330 A for 24 h.
As microorganisms grow and metabolize nutrients, large molecules change to smaller ones in liquid phase and cause a change in electrical conductivity and resistance in the liquid and at the interphase of electrodes. The bacterial level associated with this change in conductance is called the bacterial threshold level. This time is called “impedance detection time” (IDT) that is usually inversely proportional to the log number of bacteria in the sample. Usually, IDT is not visible until the number of bacteria approaches 106 - 107 CFU/mL. Many studies indicate that detection time is related to the initial population. Thus, when a sample has a large initial population, the IDT will be shorter than that of a sample with a smaller initial population (12, 13).
3.4. Statistical Analysis
Data analysis was performed using nonparametric McNemar’s test and Chi-square test in SPSS version 16. P value less than 0.05 was considered statistically significant.
4. Results
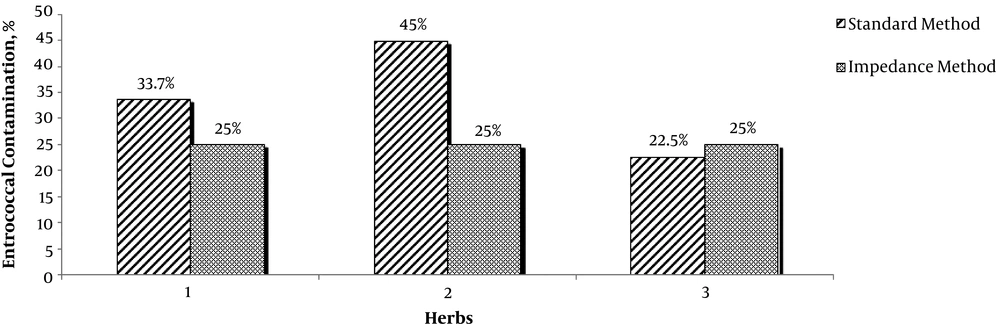
The results of the enterococci detection by using the two methods in the 40 traditional and 40 industrial samples are shown in Table 1 and Figure 1. Considering herb type, contamination rate was higher in fennel and valerian. The maximum and minimum detection times in the impedance method for the total samples were 0.66 and 21.11 hours, respectively, which is a considerable speed compared to the minimum time necessary for enterococci detection in the standard method (5 to 6 days). Considering the industrial and traditional herbs, the maximum and minimum times for detection in the impedance method were 6.71 and 21.11 hours in industrial and 0.66 and 14.46 hours in traditional samples, respectively. These results showed higher contamination in traditional medicinal herbs. However, Chi-square test did not show any significant differences between industrial and traditional samples using the two methods (P > 0.05).
| Method | Traditional Samples, N = 40 | Industrial Samples, N = 40 | Total Samples, N = 80 |
|---|---|---|---|
| Impedance | |||
| Negative | 30 | 30 | 60 |
| Positive | 10 | 10 | 20 |
| Standard | |||
| Negative | 22 | 31 | 53 |
| Positive | 18 | 9 | 27 |
The Contaminated Samples with the Standard and Impedance Methods
Comparing the obtained results of standard and impedance methods in detecting enterococci by the non-parametric McNemar’s test, we found no significant difference between the two methods (P > 0.05). Also, sensitivity and specificity of the impedance method in detecting enterococci were 51.9% and 88.7%, respectively, and the kappa value between the two methods was equal to 0.43 (P < 0.001; Table 2). Accordingly, the agreement between the two methods for all the samples was 76.2%. These results were also observed in industrial samples with the sensitivity and specificity of 55.6% and 83.9%, respectively, and in traditional samples with the sensitivity and specificity of 50% and 95.5%, respectively. Also, the agreement rates between the two methods for industrial and traditional samples were 77.5% and 75%, respectively. The results of traditional samples indicated significant differences between the two methods using McNemar’s test (P < 0.05).
5. Discussion
Herbal medicines, as the first choice in self-treatment, are popular in the general population worldwide, and a large number of modern drugs are derived from them. Although it is commonly believed that most herbal products are safe, some herbs could be toxic and harmful with undesirable side effects. Thus, the safety of herbal products is an important public health concern. Medicinal plants and herbal products normally carry a number of soil organisms. Additional microbial contaminations may result from poor methods of harvesting, cleaning, drying, handling, transportation, processing, and storage (14).
Fecal streptococci, including enterococci, have been considered an alternative potential indicator of fecal pollution of foods because they are more resistant than coliforms (primary fecal indicator) to most environmental insults (4).
Contamination of medicinal herbs with antibiotic-resistant bacterial strains is hazardous. In a study performed at Czech Republic, 250 enterococcal isolates from different types of foods were analyzed based on virulence and antibiotic resistance. It was found that 88.4% of isolates had at least one gene encoding a virulence factor. Antibiotic resistance analysis suggested that all the isolates had cross-resistance against different antibiotics (15). Separation of enterococci from plants in different regions has been reported previously (16-18). One of the barrier to acceptance of medicinal plants is the absence of a standard quality control index (14). Therefore, similar concepts of quality control and quality guarantee existing in food regulations should be applied to herbal products as well, because they have the same risk of contamination.
Since traditional microbiological methods are time-consuming and labor-intensive, rapid and automatic methods are valuable in applied microbiology (12, 19). Impedance measurement as a faster technique can replace the conventional plating methods for quality control (8). Many efforts have been made to apply the impedance method for the detection of different bacteria in different samples. In a study, psychrophilic and mesophilic bacterial loads of ice stored fresh silver pomfret were evaluated by two conventional and impedimetric methods. The impedance detection times were highly correlated with psychrophilic (r = -0.9614) and mesophilic (r = -0.9547) bacterial loads (13).
Grossi et al. evaluated total microbial density in all types of ice creams sold in Bellona through standard and impedance methods. They stated that impedance method could be used as a reliable, applicable, and easy method for evaluating the quality of ice creams (20). In this study, based on the minimum time spent for obtaining results in impedance method, more contamination was found in traditional medicinal plants, although no significant differences were observed between industrial and traditional samples (P > 0.05). Also, based on Table 1, positive results in traditional samples using the standard method were more than the impedance method. It seems that in traditional samples, because of the high possibility of cross-microbial contamination, there is a probability of obtaining false positive results. Chemical tests are used as reference methods, such as resistance to bile hydrolysis of esculin, TTC reduction, and formazan production in the identification of Enterococci and Streptococci of group D. However, Gram-positive bacteria such as Listeria monocytogenes may cause false-positive results in these tests; therefore, it seems that more diagnostic and molecular tests are required to confirm contamination to enterococci.
| Method | Standard | Total Samples | |
|---|---|---|---|
| Positive | Negative | ||
| Impedance | |||
| Positive | 14 | 6 | 20 |
| Negative | 13 | 47 | 60 |
| Total samples | 27 | 53 | 80 |
Comparison of Standard and Impedance Methods
Despite previous studies on enterococcal contamination in different types of feed, there is a lack of data on contamination of medicinal plants by enterococci. Analyzing microbial quality of plants, especially medicinal plants, based on nutritional habits and increasing attention to continuous analysis of contaminants’ levels in foods, water, and plants are required for sanitary control and management. In most countries, herbal products are sent into the market without suitable scientific evaluations and without any safety and toxicology studies (14). It is needed to ensure that the distributed herbal medicines are in accordance with microbiological standards, and attaining this goal is possible by using rapid microbial tests in order to monitor the microbial quality of herbal medicines.