1. Background
Organisms such as bacteria, viruses, and some parasites may be active even in the presence of antibiotics and antiviral/antimalarial agents. This causes treatments to be ineffective and infections to distribute to other parts of the body. Antimicrobial resistance caused by the application of antimicrobial drugs has encouraged researchers to search for new antimicrobial drugs (1). A disadvantage of organic compounds used for asepsis is their toxicity for human cells; thus, attention is increasing to inorganic disinfectants such as metal oxide nanoparticles (2).
Flavonoids, saponins, alkaloids, and phenolic compounds are the most considerable bioactive phytochemical constituents. The use of phytochemicals and plant extracts (with antibacterial properties) can be of great significance in therapeutic treatments. Nanotechnology has shown high potential for a variety of applications, e.g., treatment of resistant microbial infections (3). Nanotoxicity acceleration by oxidative stress is attributed to the presence of free radical species following NPs administration (4). Great interest has recently been devoted to introducing natural materials as new antibacterial agents. Thus, various traditional medicinal plant extracts have been applied. The effectiveness of traditional herbs against microorganisms has led to the application of plants in modern medicine to attain new principles (5). The extensive use of synthetic drugs causes increased side effects (6). One of the main herbs that seems to be good for bacterial infection treatment is Echinacea purpurea that is cultivated and collected in Nourabad, Fars. This herb has been used as an ingredient in traditional foods, supplements and certain candies. There is clinical evidence about its effectiveness in common cold and some inflammations (7). The other herb is Pulicaria undulate that grows in natural habitats in Fars and has a dense, white-woolly branched stem (8). Its extract is full of phenolic and monoterpene hydrocarbons and a few sesquiterpene hydrocarbons (9).
2. Objectives
Due to the presence of many antimicrobial-resistant pathogens, researchers have attempted to replace antimicrobial drugs with different nanoparticles, medical plants, and their combinations. This study aimed to assess the antibacterial, antifungal, and DNA cleavage (against E. coli) effects of Ni:FeO(OH) nanowires and NiS nanoparticles with/without Pulicaria undulate and Echinacea purpurea extracts.
3. Methods
3.1. Preparation of Plant Extracts and Nanoparticles/Nanowires
Pulicaria undulate and Echinacea purpurea were collected at the flowering stage in Darab, Fars, Iran, in July 2014. For the preparation of ethanol extract, 50 g of sieved plant powder was mixed with ethanol 80% so that it covered the powder. The mixtures were placed on a shaker for 24 hours in a dark room at room temperature. The supernatant was filtered through Whatman filter paper No. 2 and allowed to evaporate in vacuum condition at 45°C for 24 hours using a rotary evaporator (Biotech, Germany). The condensed extracts were incubated at 50°C for 12 hours. The weights of the obtained dried plants were measured and stored in suitable conditions (4°C) for further use. Then, Gas Chromatography-Mass Spectrometry (GC-MS) was performed on extracts. By using an Agilent-7890A chromatograph interfaced with an Agilent-5975C mass spectrometer, the GC-MS analyses were performed in a scan time of 0.5 second, ionization voltage of 70 eV, and the scan range of 40 - 400 Da while the device was equipped with capillary column HP-5. The temperatures of the detector and injector were 250°C. The oven temperature was held at 260°C for 3 minutes and helium was the carrier gas (1.0 mL/min) at a split of 1:50. The initial temperature was 60°C for 5 minutes that programmed to increase from 60 to 260°C at a rate of 50°C/min. The relative percentage of components of each extract was expressed as the percentage with peak area normalization (10). The X-ray and Field Emission Scanning Electron Microscopy analyses were done on NiS particles and FeO(OH) nanowires (11). To prepare NiS particles, 30 mL of 1.0 M Nickel sulfate [(NiSO4,6H2O)] solution and 1.0 M triethylamine [(C2H5)3N] solution were thoroughly mixed and 2 mL of concentrated sulfuric acid was added to remove the produced precipitate. For the synthesis of Ni-doped Ferric Oxyhydroxide (FeO(OH)) nanowires, we prepared a reaction solution for loading Ni-doped FeO(OH) nanowires (Ni:FeO(OH)-NWs), as follows: 0.018 mmol iron (ii) sulfate, 0.0190 mmol nickel (ii) sulfate, and 0.021 mmol NH4Fe (SO4)2 were dissolved in 20 mL deionized water using 6 mL concentrated sulfuric acid solution and diluted to 150 mL at pH of 2.35 by addition of deionized water.
3.2. Bacterial Strains
Bacterial strains were provided from the Institute of Standard and Industrial Research of Iran. We used two Gram-negative bacteria (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 9027), two Gram-positive bacteria (Staphylococcus aureus ATCC 6538 and Bacillus subtilis ATCC 6633), one mold (Aspergillus oryzae), and one yeast (Candida albicans). Bacterial strains were prepared based on the instructions of the company, cultured on Mueller-Hinton agar (Merck, Germany) plates, and incubated (Memert, Germany) at 37°C for 24 hours before use.
3.3. Antibacterial Activity (In Vitro)
The antimicrobial activity of materials was investigated against E. coli, B. subtilis, S. aureus, and P. aeruginosa using the disc diffusion method. Typically, 100 µL of each bacterial culture containing 0.5 × 106 (0.5 McFarland turbidimetry) colony-forming units was occulted and spread onto the Muller Hinton Agar medium with a sterile swab (12). All compounds were dissolved in DMSO (100, 50, 25 mg/mL, and equal volumes for combination; e.g., for the preparation of 100 mg/mL concentration, 50 mg/mL of the extract was mixed with 50 mg/mL of nanoparticles in a 1000 µL final volume). Then, sterile paper disks (6 mm, 50 μL) soaked at different concentrations were placed on the medium and incubated at 37°C for 24 hours. We used DMSO and two antibiotics (gentamicin, vancomycin) as positive and negative controls, respectively (13).
3.4. Antifungal Activity (In Vitro)
The antifungal properties of materials were investigated against Candida albicans and Aspergillus oryzae. The experiments were carried out using the disc diffusion technique. In a typical procedure, the prepared discs (at concentrations of 100, 50, and 25 mg/mL per disc in DMSO solution) were placed on MHA and Saboroud Dextrose Agar (SDA) media (Merck, Germany), which had been occulted with 100 µL (105 CFU/mL) of fungal suspensions. The plates of C. albicans (isolated from patients) and A. oryzae ATCC 5164 were incubated at 32°C for 24 hours and 25°C for 72 hours, respectively. We used DMSO and Clotrimazole as negative and positive controls, respectively (13).
3.5. Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) and MBC were determined by utilizing the broth dilution assay method. For the description of MICs of nanoparticles and extracts against bacteria, 10-fold serial dilutions of compounds or extracts were prepared, as follows: 500 mg/mL of extracts or nanomaterials were prepared and 100 μL of these suspensions were added to 900 μL of DMSO. The serial dilution preparations continued. Then, 100 μL of a fresh bacterial culture (0.5 × 106 CFU), 200 μL of each suspension (equal volumes for combination: 100 μL of extract + 100 μL of nanoparticles), and 700 μL of Muller Hinton broth were transferred to sterile test tubes. Bacterial growth was examined after 24 hours of incubation at 37°C (14). The MICs of the materials were taken as the lowest concentrations that inhibited bacterial growth as observed in tubes.
3.6. Minimum Bactericidal Concentration
The minimum bactericidal concentration (MBC) is the lowest concentration of extracts and compounds that killed all microorganisms. For the detection of MBC, each of the bacterial cultures in Muller Hinton Broth was occulted on MHA medium using a loop, incubated for 24 hours at 37°C, and evaluated for bacterial growth (14).
3.7. DNA Cleavage Experiment
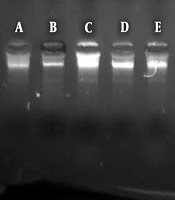
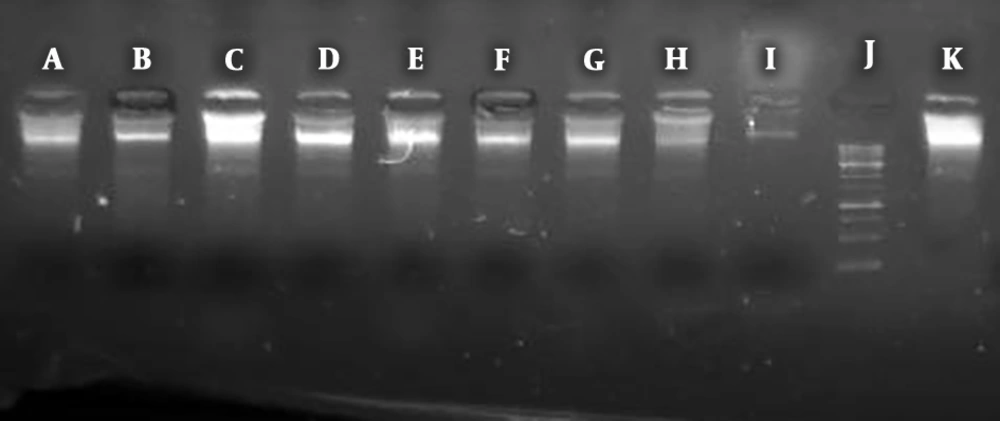
Luria-Bertani medium (Merck, Germany) was used for culturing E. coli. First, 10 mL of the medium was prepared, inoculated with bacteria, and incubated at 37°C for 24 hours. The bacteria containing chromosomal DNA (1.5 mL) was centrifuged for 7 minutes at 7000 g (Hastaran, Iran). The DNA extraction was performed based on the Gram-negative DNA extraction kit protocol (CINNAPURE, Iran) and chromosomal DNA was stored at -40°C for tests. Samples (5 mg/mL) of extracts, nanoparticles/nanowires, or combinations were added to the extracted chromosomal DNA (5 μg) and incubated for 2 hours at 37°C. After incubation, 10 μL of DNA sample was added to 6X loading buffer and loaded into electrophoresis wells (1% gel stained with 10 μg/mL of Ethidium Bromide). After passing 100 V (constant voltage) for about 50 minutes, the bands were observed, compared with the positive control (pure DNA diluted in DEPCE water), and denatured using equal volumes of chromosomal DNA and 4% H2O2 (11).
4. Results
4.1. Identification of Components
The components were identified by matching the mass spectra of components with Wiley Registry of Mass Spectral Data, 7th edition (Agilent Technologies, Inc.) and National Institute of Standards and Technology 08 MS (NIST) library data. The identification was confirmed by comparison of retention indices with data in the literature (15). Twenty-two different components were specified in Pulicaria undulate extract. The identified components, with their relative percentages and Kovats indices, are given in Table 1. The main constituents of Pulicaria undulate extract were nonadecane (13.8%), methyl jasmonate (12.1%), 1-S-cis-calamenene (10.1%), and 26,26-Dimethyl-5,23-ergostadien-3.beta.-ol (6.4%). The GC analysis for Echinacea purpurea showed 21 different components (Table 2). The main constituents of Echinacea purpurea volatile oil were phytol (17.7%), germacrene D (17.7%) trans-caryophyllene (7.5%), and neophytadiene (7.5%).
| No. | Components | KI | Percent |
|---|---|---|---|
| 1 | α- terpinene | 1025 | 1.7 |
| 2 | γ- terpinene | 1071 | 2.1 |
| 3 | Cis- sabinene hydrate | 1075 | 4.1 |
| 4 | Linalool | 1100 | 2.4 |
| 5 | 4-terpineole | 1181 | 5.3 |
| 6 | α-Terpineol | 1196 | 4.6 |
| 7 | Dodecane | 1204 | 1.9 |
| 8 | β.-Citronellol | 1233 | 2.0 |
| 9 | trans-Geraniol | 1258 | 2.1 |
| 10 | Tetradecane | 1404 | 1.0 |
| 11 | neryl 2-methylpropanoate | 1494 | 4.5 |
| 12 | Hexadecane | 1601 | 1.2 |
| 13 | Methyl jasmonate | 1644 | 12.1 |
| 14 | Xanthoxylin | 1677 | 1.5 |
| 15 | 1 S-cis- calamenene | 1697 | 10.1 |
| 16 | Heptadecane | 1702 | 5.1 |
| 17 | 7-Acetyl-2-hydroxy-2-methyl-5- sopropylbicyclo[4.3.0] nonane | 1744 | 4.8 |
| 18 | 26,26-Dimethyl-5,23-ergostadien-3.beta.-ol | 1762 | 6.4 |
| 19 | Ledene oxide-(I) | 1836 | 3.2 |
| 20 | 1-Nonadecene | 1875 | 2.6 |
| 21 | Nonadecane | 1902 | 13.8 |
| 22 | Hexadecanoic acid, ethyl ester | 2009 | 2.8 |
| Total percentage | 95.3 |
| No. | Components | KI | Percent |
|---|---|---|---|
| 1 | Eugenol | 1363 | 4.0 |
| 2 | β-Elemene | 1397 | 1.0 |
| 3 | trans-Caryophyllene | 1425 | 7.5 |
| 4 | γ-Elemene | 1434 | 2.3 |
| 5 | α-Humulene | 1458 | 2.3 |
| 6 | Germacrene D | 1486 | 17.7 |
| 7 | δ-Cadinene | 1527 | 2.3 |
| 8 | 1,5-epoxysalvial-4(14)-ene | 1570 | 1.2 |
| 9 | (+) Spathulenol | 1581 | 1.6 |
| 10 | (-)-Caryophyllene oxide | 1586 | 1.2 |
| 11 | Hexadecane | 1600 | 0.9 |
| 12 | α-Cadinol | 1657 | 3.6 |
| 13 | Eudesma-4,11-dien-2-ol | 1690 | 3.2 |
| 14 | 7-Acetyl-2-hydroxy-2-methyl-5-isopropylbicyclo[4.3.0]nonane | 1741 | 1.0 |
| 15 | Tricyclo[4.4.0.02,7]dec-3-ene-3-methanol, 1-methyl-8-(1-methylethyl)- | 1760 | 1.0 |
| 16 | Octadecane | 1800 | 1.4 |
| 17 | Neophytadiene | 1838 | 7.5 |
| 18 | δ.-Fenchane | 1903 | 1.4 |
| 19 | Hexadecanoic acid | 2005 | 15.5 |
| 20 | Phytol | 2108 | 17.7 |
| 21 | Ethyl linoleate | 2164 | 1.7 |
| Total percentage | 96 |
4.2. Antibacterial and Antifungal Activities (Disc Diffusion Method)
Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa, and Staphylococcus aureus had the least resistance to Pulicaria undulate and NiS, Echinacea purpurea and Ni-doped FeO(OH), Pulicaria undulate, and Echinacea purpurea and Ni-doped FeO(OH), respectively. Among the mixtures, the combination of Echinacea purpurea and Ni:FeO(OH)-NW showed the most effect on Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus. Among extracts, Pulicaria undulate extract showed more antibacterial effects than Echinacea purpurea against all bacteria. Concerning nanostructures, Ni:FeO(OH)-NW had more effects on Gram-positive bacteria and NiS-NP showed antibacterial effects on Gram-negative bacteria. The most effect against Candida albicans was related to the combination of Pulicaria undulate and NiFeO(OH)-NW. None of the materials showed antifungal effects against Aspergillus oryzae (Table 3).
| Samples | Bacteria | Fungi | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli (mg/mL) | Pseudomonas aeruginosa (mg/mL) | Bacillus subtilis (mg/mL) | Staphylococcus aureus (mg/mL) | Candida albicans (mg/mL) | |||||||||||
| 100 | 50 | 25 | 100 | 50 | 25 | 100 | 50 | 25 | 100 | 50 | 25 | 100 | 50 | 25 | |
| Pulicaria undulate | 18.22 | 14.00 | 11.14 | 22.40 | 13.30 | 11.16 | 11.60 | 11.00 | 7.30 | 13.00 | 10.00 | 9.00 | 21.00 | 15.38 | 11.28 |
| Echinacea purpurea | 18.00 | 10.00 | 8.00 | 10.30 | 10.00 | 9.00 | 8.20 | 8.00 | 7.00 | 13.00 | 12.00 | 8.22 | 18.00 | 10.20 | 9.00 |
| Ni-doped FeO(OH) | 10.20 | 10.00 | 8.00 | 13.68 | 10.14 | 8.20 | 10.22 | 9.00 | 7.66 | 9.00 | 6.00 | 6.00 | 11.00 | 9.00 | 8.40 |
| NiS | 12.00 | 12.00 | 8.00 | 19.00 | 13.00 | 8.80 | 9.00 | 8.50 | 7.18 | 7.42 | 7.00 | 7.00 | 11.42 | 9.22 | 7.36 |
| Pulicaria undulate and Ni-doped FeO(OH) | 18.60 | 11.60 | 8.44 | 17.60 | 16.58 | 9.00 | 9.40 | 8.52 | 7.26 | 9.60 | 8.52 | 7.66 | 24.00 | 12.00 | 9.28 |
| Pulicaria undulate and NiS | 23.00 | 10.20 | 7.00 | 17.00 | 14.00 | 12.54 | 10.00 | 8.16 | 7.16 | 12.52 | 9.00 | 7.80 | 21.50 | 12.30 | 8.28 |
| Echinacea purpurea and Ni-doped FeO(OH) | 14.14 | 11.00 | 9.70 | 19.00 | 9.30 | 8.40 | 17.58 | 9.80 | 8.00 | 20.00 | 11.00 | 10.70 | 23.00 | 15.00 | 13.30 |
| Echinacea purpurea and NiS | 13.30 | 9.00 | 8.40 | 10.30 | 9.20 | 9.00 | 14.20 | 11.00 | 8.10 | 14.00 | 10.00 | 8.20 | 15.10 | 13.00 | 10.00 |
| Gentamycine (10 μg/disc) | 20.00 | 20.60 | 23.08 | 24.32 | - | ||||||||||
| Vancomycine (30 μg/disc) | 21.00 | 20.00 | 25.00 | 22.40 | - | ||||||||||
| Clotrimazole (100 μg/disc) | - | - | - | - | 20.10 | ||||||||||
4.3. Antibacterial and Antifungal Activities (Dilution Method)
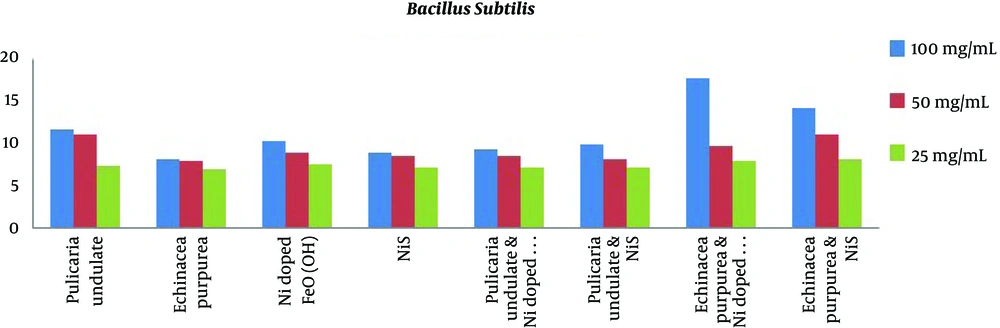
The results of MIC and MBC are presented in Table 4 and Figure 1. The results of MIC showed Echinacea purpurea and Pulicaria undulate had the highest antibacterial effects (lowest concentration) against E. coli (0.78 mg/mL). On the other hand, the results of MBC showed Echinacea purpurea and Pulicaria undulate had the highest antibacterial effects (lowest concentration) against E. coli (0.78 mg/mL and 1.56 mg/mL, respectively). Among the mixtures of materials, the mixture of Echinacea purpurea and Ni:FeO(OH)-NW showed the most antibacterial effects at the lowest concentration against Pseudomonas aeruginosa and Staphylococcus aureus. The MIC of the combination of Echinacea purpurea and Ni-doped FeO(OH) was 0.195 mg/mL for Staphylococcus aureus. On the other hand, the minimum bactericidal concentration of the combination of Pulicaria undulate and NiS against E. coli was 0.78 mg/mL.
| Samples | Escherichia coli | Pseudomonas aeruginosa | Bacillus subtilis | Staphylococcus aureus | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Pulicaria undulate | 0.78 | 1.56 | 25 | 50 | 25 | 25 | 6.25 | 25 |
| Echinacea purpurea | 0.78 | 0.78 | 25 | 50 | 25 | 50 | 12.5 | 25 |
| Ni-doped FeO(OH) | 25 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| NiS | 50 | 50 | 0.39 | 0.39 | 25 | 50 | 50 | 50 |
| Pulicaria undulate and Ni-doped FeO(OH) | 0.39 | 0.78 | 50 | 50 | 25 | 50 | 25 | 50 |
| Pulicaria undulate and NiS | 0.195 | 0.195 | 25 | 50 | 25 | 50 | 25 | 25 |
| Echinacea purpurea and Ni doped FeO(OH) | 50 | 50 | 0.195 | 0.78 | 0.39 | 1.56 | 0.195 | 0.195 |
| Echinacea purpurea and NiS | 50 | 50 | 25 | 50 | 12.5 | 12.5 | 25 | 50 |
4.4. DNA Cleavage Experiment
The most DNA cleavage was related to the mixture of NiS-NP and Pulicaria undulate while the weakest compound in this regard was Echinacea purpurea (Figure 2).
5. Discussion
In the present study, we evaluated the antibacterial effects of ethanolic extracts of Pulicaria undulate and Echinacea purpurea. We showed that they were effective against several bacteria. Also, the antibacterial effects of Pulicaria undulate and Echinacea purpurea extract with/without Ni:FeO(OH)-NW and NiS-NP were assessed. All compounds had antibacterial effects although the mixture of Pulicaria undulate and NiS-NP showed the most effects on Escherichia coli (at a concentration of 100 mg/mL).
Mixing extracts with nanostructures (synergistic effects) often influenced Gram-negative bacteria more than Gram-positive bacteria; this can be related to the structure of the cell wall and the difference between the positively charged nanoparticles and negatively charged bacteria. Furthermore, the NPs acted as electromagnets and adsorbed microbes because NPs could bind to the cell surface. Phytol, (3,7,11,15-tetramethylhexadec-2-EN-1-OL), as one of the products in chlorophyll metabolism in plants, is one of the main compounds in Echinacea purpurea; it is also one of the most substantial di-terpenes. It possesses both anticancer and antimicrobial activities (7). The results of research by Izadi et al. have shown that the essential oil of Echinacea purpurea plant has antimicrobial effects and can be used as an alternative to synthetic antibiotics (16). In another research done by Hajipour et al., the effects of nanoparticles like ZnO, NiO, and Sb2O3 against B. subtilis, E. coli, and S. aureus were studied. The results showed that the toxicity of ions was not significant and the natural toxic properties of heavy metals led to the toxicity of metal oxide nanoparticles (2). The DNA cleavage experiment confirmed that the mixture of Pulicaria undulate and NiS-NP had the most effect on chromosomal DNA of E. coli Additionally, compounds had no effect on Aspergillus oryzae and the most effects were on Candida albicans (the mixture of Pulicaria undulate and Ni:FeO(OH)-NW) at 100 mg/mL. Arif Khan et al. characterized the pharmacology of methanol extract of Calligonum polygonoides. Growth inhibition of Aspergillus niger was measured. Their study showed C. polygonoides had noticeable antifungal, antioxidant, and cytotoxic bioactive compounds (17). Antimicrobial activities of Piper nigrum, Albizia lebbeck, and Calligonum polygonoides were screened through the agar tube dilution method, too (18). Based on these results, the growth of Gram-negative bacteria (E. coli) and Gram-positive bacteria (S. aureus) was significantly inhibited by C. polygonoides. Some researchers report that phytol is a significant compound in these extracts and is one of the most substantial di-terpenes produced in chlorophyll metabolism in plants. As phytol is one of the main compounds in Echinacea purpurea, it seems that some antimicrobial properties were caused by this compound. As a result, the combinations of Pulicaria undulate and Echinacea purpurea extracts with nanostructures were effective to eliminate bacteria. Thus, combinations of plant extracts and nanoparticles can be new options for the treatment of infectious diseases due to possessing synergistic effects.