1. Background
Mycoendophytes (Endophytic fungi), commonly defined as fungi spending, in the whole or a part of their lifecycle colonizing the internal tissue of healthy plants causing no apparent symptoms of disease (1). The research in this field gained a great interest in early 1990’s by discovering the ability of an endophytic fungus (Taxomyces andreanae) isolated from Taxus baccata (a Pacific yew tree) to produce paclitaxel (Taxol®: a multibillion-dollar anticancer drug) as its host plant. That discovery evolved the interest in endophytes as potential new sources for therapeutic agents (2). Recently, endophytes are recognized as a source of a variety of new biologically active compounds potentially useful for human medicine (3).
Medicinal plants as a source of bioactive compounds are well known since the ancient time. One limiting factor in this process is the plant availability (2). Recently, focus is shifted to plant microbiome especially endophytic fungi, which may produce similar if not the same bioactive compounds as their hosts (2). Moreover, it is suggested that endophytes of medicinal plants that populate distinct and unique habitats may represent a promising source for new bioactive compounds where the organisms in such habitats should adapt with extreme living conditions such as cold, heat, and multitudinous competing organisms (4).
2. Objectives
The current study aimed at isolating a group of endophytic fungi of medicinal plants that populate different habitats including distinct and unique habitats as a trial to obtain new microbial isolates producing novel natural bioactive compounds.
3. Methods
3.1. Plants and Sampling Area
Samples of 10 medicinal plants (Capparis spinosa, Euphorbia helioscopia, Nepeta septemcrenata, Peganum harmala, Plantago sinaica, Astragalus annularis, Avicennia marina “mangrove”, Hyoscyamus muticus, Calotropis procera, and Moringa oleifera) were collected from different geographical locations in Egypt (Saint-Catherin, Ras-Mohammad, Nile-Valley).
3.2. Sampling
Leaf, bark, flower, and fruit samples (if available) of mature healthy plants were collected, put in plastic bags, and kept in ice box until transported to the laboratory (1, 5).
3.3. Fungal Endophytes Isolation
Samples were washed, sterilized, and fractionated according to the method described by Garyalis et al. (5). Sample pieces were cultured on potato-dextrose agar (PDA), Czapek-Dox’s agar, and malt-yeast agar plates supplemented with ampicillin (50 µg/mL) and incubated at 28°C for 21 days. Isolates were identified at least to genus level depending on their morphological characteristics and the microscopic examination with the aid of the identification keys provide by the following references (6-9). Also, eight isolates were identified in Mycological Center, Assiut-University, Egypt.
3.4. Fermentation and Crude Extract Preparation
Fungal endophytes were cultivated on PDA plates for 14 days at 25°C. The culture medium and the fungal mycelium of three plates were cut into small squares and soaked in 150 mL absolute ethanol (El Nasr, Egypt) in 500 mL beaker tightly covered and incubated under stirring conditions at 15°C for 24 hours. Solvent was filtrated using Whatman filter paper No. 1; then the solvent was evaporated by rotary evaporator at 48°C and 150 rpm to collect the crude extract ((10) with slight modifications).
3.5. Bioactivity Assaying
3.5.1. Antimicrobial Assay
3.5.1.1. Antibacterial
Antibacterial activity of crude extracts was investigated against three Gram-positive bacteria (i.e., Micrococcus luteus clinical isolate, Streptococcus pneumoniae clinical isolate, and Staphylococcus aureus ATTC 25923) and three Gram-negative bacteria (E. coli ATTC 25922, Klebsiella pneumoniae ATTC 700603, Pseudomonas aeruginosa ATTC 27853). The assay was carried out by Kirby-Bauer disc diffusion method (11). Muller-Hinton agar plates (9 cm diameter containing 20 mL medium) were inoculated with 100 µL bacterial suspension (0.2 OD). The filter paper discs (0.6 cm) loaded with 200 µg of crude extract were placed on the agar plate surface and incubated at 4°C overnight for compounds diffusion and then incubated at 37°C for 24 hours. The antimicrobial activity was detected by measuring the inhibition zone diameter.
3.5.1.2. Antifungal
Antifungal activity of crude extracts was tested against Candida albicans (clinical isolate) using the previous disc diffusion method. Antifungal activities against Fusarium solani were tested by calculating the mycelial percent growth inhibition as follows:
Where, G1 is fungal-growth-diameter in PDA control plate and G2 is fungal-growth-diameter in PDA plate supplemented with crude extract (80 µg/mL) (12).
3.5.2. Cytotoxicity Assay
Cytotoxicity was determined by MTT assay method (13) against normal cell line MRC-5 ATCC CCL-171 and cancer cell line MCF-7 ATCC HTB-22. Different concentrations of extracts (the tested concentration range was 0.31 - 31 mg/mL depending on the productivity of the crude extract by the isolate and its toxicity) were added to the cell monolayer grown on a 96-well tissue culture plate at a concentration of 105 cell/mL using RPMI medium, then incubated for 48 hours at 37°C in an incubator humified with 5% CO2. The culture medium was decanted, and the plate wells were washed twice with phosphate buffer saline without Ca++ and Mg++. A total of 50 µL of MTT reagent (made up in medium to a final concentration of 0.5 mg/mL) was added to the wells and incubated in dark for four hours; 100 µL of di-methyl sulfoxide (DMSO) was added to wells to solubilize the purple crystals of formazan. Absorbance (Ab) was measured at 570 nm by microplate ELISA reader. Percent cell growth inhibition was calculated as follows:
IC50 value was calculated by plotting dose response curve.
4. Results and Discussion
Fifty-four endophytic fungal strains were isolated from the screened medicinal plants collected from different geographical Egyptian locations (Figure 1). Forty-eight isolates were identified, at least to genus level, depending on their morphological and microscopic features. Six isolates were non-sporulating strains; thus they were considered as mycelia sterilia isolates (Table 1). All the identified isolates belonged to Ascomycota, which agreed with the previous observations indicating that most of isolated endophytic fungi belong to ascomycetes (1, 14). The present study results showed that Aspergillus genus was the most common isolate followed by Eladia “Penicillium related strains (7)” and Cladosporium. Aspergillus, Penicillium, and Cladosporium were also reported among the dominant endophytic isolates obtained in many other studies (15-17).
| Host Plant | Sampling Location | Season (mn) | Tissue | Isolated Endophyte | |
|---|---|---|---|---|---|
| Identification | Isolate Code | ||||
| Capparisspinosa | Saint Catherine (Sinai) | May 2014 | Bark | Eladia sp. | 7-H |
| Eladia sp. | 8-I | ||||
| Eladia sp. | 9-J | ||||
| Eladia sp. | 10-L | ||||
| Eladia sp. | 11-M | ||||
| Leaf | Eladia sp. | 3-C | |||
| Cladosporium sphaerospermum | 5-E | ||||
| Alternaria sp. | 6-F | ||||
| Euphorbia helioscopia | Saint Catherine (Sinai) | May 2014 | Bark | Cladosporium sp. | 18-T |
| Penicillium sp. | 19-U | ||||
| Penicillium miczynskii | 25-III | ||||
| Leaf | Eladia sp. | 14-P | |||
| Gliocladium roseum | 15-Q | ||||
| Cladosporium sp. | 16-R | ||||
| Penicillium miczynskii | 17-S | ||||
| Cladosporium sp. | 26-IV | ||||
| Aspergillus sp. | 27-(v) | ||||
| Flower | - | ||||
| Nepetaseptemcrenata | Saint Catherine (Sinai) | May 2014 | Bark | Alternaria sp. | 12-N |
| Dark sterile mycelium | 13-O | ||||
| Trichoderma sp. | 20-V | ||||
| Alternaria sp. | 21-W | ||||
| Dark sterile mycelium | 22-X | ||||
| Trichoderma sp. | 23-Y | ||||
| Dark sterile mycelium | 24-II | ||||
| Trichoderma sp. | 30-XII | ||||
| Dark sterile mycelium | 31-XIII | ||||
| Leaf | Aspergillus niger | 61-61 | |||
| Peganumharmala | Saint Catherine (Sinai) | May 2014 | Bark | - | - |
| Leaf | - | - | |||
| Fruit | Cladosporium sp. | 28-(X) | |||
| Plantagosinaica | Saint Catherine (Sinai) | May 2014 | Bark | - | - |
| Flower | Alternaria sp. | 29-XI | |||
| Eladia sp. | 4-D | ||||
| Astragalusannularis | Saint Catherine (Sinai) | May 2014 | Bark | Eladia sp. | 1-A |
| Aspergillus terreus | 2-B | ||||
| Leaf | - | - | |||
| Avicennia marina | Ras-Mohammed (Red Sea coast - Sinai) | April 2016 | Bark | Cladosporium sp. | 56-*2 |
| Leaf | Aspergillus sp. | 52-ML1 | |||
| Pochonia suclosporia | 53-ML2 | ||||
| Flower | Chaetomium globosum | 54-MF1 | |||
| Dark sterile mycelium | 55-MF2 | ||||
| Hyoscyamusmuticus 2 | Cairo (Helwan University) | September 2015 | Bark | Nigrospora sp. | 38-38 |
| Cladosporium sp. | 40-40 | ||||
| Penicillium sp. | 41-41 | ||||
| Aspergillus flavus | 45-45 | ||||
| Leaf | Nigrospora sp. | 39-39 | |||
| Hyoscyamusmuticus 1 | October 2013 | Bark | Aspergillus terreus | 35-SB | |
| Leaf | - | - | |||
| Moringaoleifera 2 | Giza | September 2015 | Bark | Curvularia pallescens | 48-48 |
| Aspergillus niger | 50-50 | ||||
| Leaf | Nigrospora sp. | 49-49 | |||
| Moringaoleifera 1 | October 2013 | Bark | Curvularia sp. | 34-34 | |
| Aspergillus flavus | 60-60 | ||||
| Calotropisprocera 2 | Cairo (El Maadi) | September 2015 | Bark | Penicillium sp. | 42-42 |
| Cladosporium sp. | 43-43 | ||||
| Aspergillus sp. | 44-44 | ||||
| Aspergillus flavus | 46-46 | ||||
| Leaf | - | - | |||
| Calotropisprocera 1 | Cairo (Helwan University) | October 2013 | Bark | Dark sterile mycelium | 36-36 |
| Leaf | Alternaria sp. | 37-37 | |||
Endophytes Isolated from Plants Collected from Different Habitats
Ethanolic extracts of these isolates were prepared to test their biological activities (Note: only 49 fungal isolates were screened where the Aspergillus niger and Aspergillus flavus isolates were excluded due to their contamination issues).
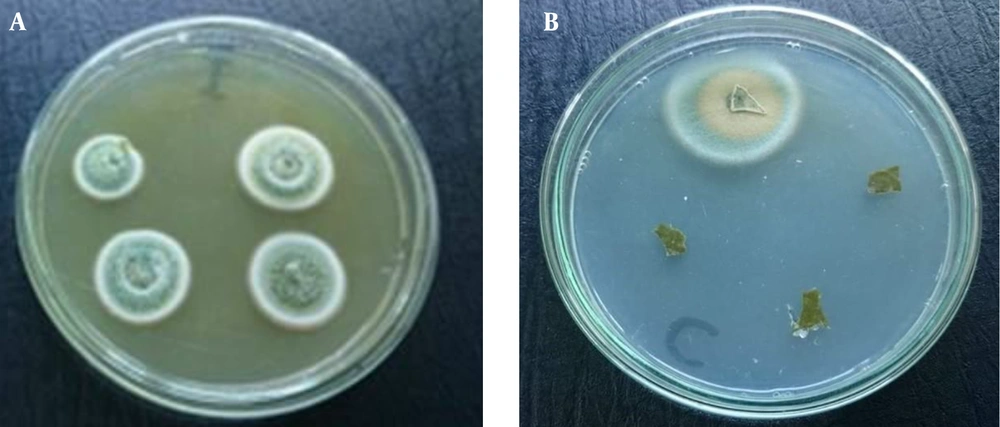
The crude extracts were screened for their antimicrobial activities. It was found that the crude extracts of only 26 isolates showed antibacterial activities at least against one tested bacterial strain and only the crude extracts of seven isolates showed antibacterial activities against both Gram-negative and -positive bacteria. On the other hand, evaluation of antifungal activity of the extracts showed that Candida albicans was resistant to all tested extracts and the extract of only one isolate (Aspergillus terreus 35-SB) showed a relatively acceptable percent growth inhibition against Fusarium solani (35.6%) (Table 2). Jalgaonwala et al., evaluated the antimicrobial activity of 142 fungal endophytes isolated from medicinal plants. It was reported that 14 endophytic fungal isolates possessed antibacterial activity (18). In another study, 160 endophytic fungi derived from Cymodocea serrulata, Halophila ovalis, and Thalassia hemprichii were screened for production of antimicrobial compounds against 10 human pathogenic microorganisms; 69% of the isolates exhibited antimicrobial activity against at least one tested strain and only seven isolates exhibited strong antimicrobial activity (19). Endophytes evolve mechanisms that allow them to protect their hosts against pathogens and compete with the microorganisms for their microhabitat (inside the plant tissue); thus they represent a good source to search for antimicrobial and antifungal agents (2).
| Isolate Code | Antibacterial Activity | Antifungal Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| Gram-Positive (Inhibition Zone, mm) | Gram-Negative (Inhibition Zone, mm) | Yeast | Mold | |||||
| M. luteusb | S. pneumoniaeb | S. aureusb | E. colib | K. pneumoniaeb | P. aeruginosab | C. albicans b | F. solanib %inhibition | |
| 1-A | - | - | - | - | - | 8.6 | - | 0 |
| 2-B | - | 10.3 | 15 | - | - | 8.6 | - | 0 |
| 3-C | - | - | - | - | - | 8 | - | 0 |
| 4-D | - | - | - | - | - | 10 | - | 0 |
| 5-E | - | - | - | - | - | - | - | 0 |
| 6-F | - | - | 8.2 | - | - | - | - | 0 |
| 7-H | - | - | - | - | - | - | - | 0 |
| 8-I | - | - | - | - | - | - | - | 0 |
| 9-J | - | - | - | - | - | - | - | 0 |
| 10-L | - | - | - | - | - | - | - | 0 |
| 11-M | - | - | - | - | - | - | - | 0 |
| 12-N | - | - | - | - | - | - | - | 0 |
| 13-O | - | - | 13.5 | - | - | 7 | - | 0 |
| 14-P | - | - | - | - | - | - | - | 0 |
| 15-Q | - | - | - | - | - | - | - | 0 |
| 16-R | - | - | - | - | - | - | - | 0 |
| 17-S | 9.3 | 7.7 | 6.7 | - | - | 8 | - | 13.8 |
| 18-T | - | - | - | - | - | 8 | - | 0 |
| 19-U | - | - | - | - | - | 8.5 | - | 0 |
| 20-V | - | - | - | - | - | 11.5 | - | 0 |
| 21-W | - | - | - | - | - | - | - | 0 |
| 22-X | - | - | 13 | - | - | - | - | 2.6 |
| 23-Y | - | - | - | - | - | - | - | 0 |
| 24-II | - | - | 9.3 | - | - | - | - | 0 |
| 25-III | 8.3 | 6.8 | - | - | - | 8.7 | - | 15.5 |
| 26-IV | - | - | - | - | - | - | - | 0 |
| 27-(V) | - | - | - | - | - | 7.3 | - | 0 |
| 28-(X) | - | - | - | - | - | 8.7 | - | 0 |
| 29-XI | - | - | 6.5 | - | - | - | - | 1.7 |
| 30-XII | - | - | - | - | - | - | - | 0 |
| 31-XIII | - | - | 7.3 | - | - | - | - | 1.7 |
| 34-34 | - | - | - | - | - | - | - | 0 |
| 35-SB | 18.3 | 10.3 | 7.3 | - | - | - | - | 35.6 |
| 36-36 | - | - | - | - | - | - | - | 0 |
| 37-37 | - | 7 | 7.3 | 7 | - | - | - | 0 |
| 38-38 | - | - | - | - | - | - | - | 0 |
| 39-39 | - | - | - | - | - | - | - | 0 |
| 40-40 | - | - | - | - | - | 7.2 | - | 0 |
| 41-41 | - | - | - | - | - | 7.7 | - | 0 |
| 42-42 | 12.7 | 6.75 | - | - | - | 8 | - | 0 |
| 43-43 | - | - | - | - | - | 7 | - | 0 |
| 44-44 | - | - | - | - | - | - | - | 0 |
| 48-48 | - | - | - | - | - | 7.5 | - | 0 |
| 49-49 | - | - | - | - | - | 7.3 | - | 0 |
| 52-ML1 | - | - | - | - | - | - | - | 0 |
| 53-ML2 | - | - | - | - | - | - | - | 0 |
| 54-MF1 | 10.5 | 6.5 | 8 | 6.5 | 7 | 7 | - | 0 |
| 55-MF2 | - | - | - | - | - | - | - | 0 |
| 56-*2 | - | - | - | - | - | - | - | 0 |
Antimicrobial Activity of Endophytic Fungal Crude Extractsa
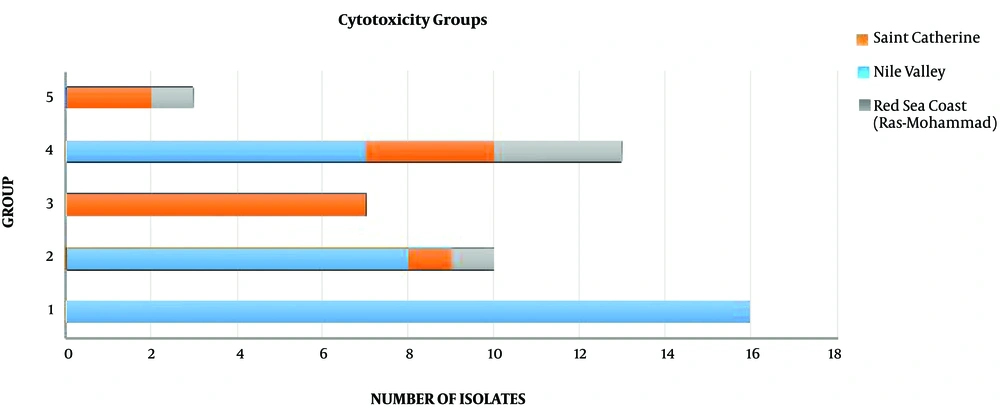
Cytotoxicity of tested crude extracts was examined against one normal cell line (MRC-5) and another cancer cell line (MCF-7) using MTT assay. According to the obtained results, the screened isolates were divided into five groups (Table 3):
The first group showed a nontoxic effect towards both the normal and the cancer cell lines. The second group showed a variable effect ranging between non- to reduced toxicity towards the normal and/or the cancer cell lines. The third group exerted a reduced toxicity towards the normal cell line, but showed a higher toxic effect against the cancer cell line. This group consisted of seven isolates (A. terreus 35-SB, Nigrospora sp. 38-38, Penicillium sp. 41-41, Penicillium sp. 42-42, Cladosporium sp. 43-43, Aspergillus sp. 44-44, and Curvularia pallescens 48-48). These isolates represented a promising source for anticancer compounds with low cytotoxicity against the normal cell lines. The fourth group showed toxic effects towards both the cancer and the normal cell lines. The final group showed toxic effects against the normal cell line and reduced toxicity towards the cancer cell line (Figure 2).
| Isolate Code | Concentration, mg/mL | %Inhibition | Toxicity | IC50, mg/mL | |||
|---|---|---|---|---|---|---|---|
| MRC-5 | MCF-7 | MRC-5 | MCF-7 | MRC-5 | MCF-7 | ||
| 1-A | 15.5 | 1.7 | 5.1 | Non-toxic | Non-toxic | ND | ND |
| 2-B | 15 | 96.2 | 70.4 | Toxic | Toxic | 9.1 | 9.5 |
| 7.5 | 34 | 52.1 | |||||
| 3.75 | 0 | 33 | |||||
| 1.88 | 0 | 0 | |||||
| 0.94 | 0 | 0 | |||||
| 3-C | 15 | 7.7 | 18.7 | Non-toxic | Reduced-toxicity | ND | ND |
| 4- D | 15.5 | 7 | 0 | Non-toxic | Non-toxic | ND | ND |
| 5-E | 15 | 6.8 | 1.5 | Non-toxic | Non-toxic | ND | ND |
| 6-F | 15.5 | 4.7 | 0 | Non-toxic | Non-toxic | ND | ND |
| 7-H | 16 | 1 | 0 | Non-toxic | Non-toxic | ND | ND |
| 8-I | 15 | 15 | 8.2 | Reduced-toxicity | Non-toxic | ND | ND |
| 9-J | 30 | 22.6 | 7.4 | Reduced-toxicity | Non-toxic | ND | ND |
| 10-L | 15 | 11.8 | 7.2 | Reduced-toxicity | Non-toxic | ND | ND |
| 11-M | 16.5 | 18.6 | 0.7 | Reduced-toxicity | Non-toxic | ND | ND |
| 12-N | 7.5 | 16.7 | 2.9 | Reduced-toxicity | Non-toxic | ND | ND |
| 13-O | 15.5 | 100 | 100 | Toxic | Toxic | ND | ND |
| 14-P | 15 | 20.9 | 15.3 | Reduced-toxicity | Reduced-toxicity | ND | ND |
| 15-Q | 5 | 9.7 | 1.6 | Non-toxic | Non-toxic | ND | ND |
| 16-R | 16 | 13.1 | 9.5 | Reduced-toxicity | Non-toxic | ND | ND |
| 17-S | 15.5 | 0 | 2.3 | Non-toxic | Non-toxic | ND | ND |
| 18-T | 5 | 9.2 | 0 | Non-toxic | Non-toxic | ND | ND |
| 19-U | 16 | 66.3 | 94.2 | Toxic | Toxic | 9.8 | 7 |
| 8 | 58 | 84.8 | |||||
| 4 | 50 | 46.6 | |||||
| 2 | 0 | 0 | |||||
| 1 | 0 | 0 | |||||
| 20-V | 16 | 100 | 91.8 | Toxic | Toxic | 5.2 | 4.6 |
| 8 | 84.1 | 90.4 | |||||
| 4 | 65 | 62.7 | |||||
| 2 | 38.2 | 59.7 | |||||
| 1 | 11.9 | 5.4 | |||||
| 21-W | 7.5 | 1.6 | 0 | Non-toxic | Non-toxic | ND | ND |
| 22-X | 31 | 100 | 91.8 | Toxic | Toxic | 0.19 | ND |
| 15.5 | 100 | 91.6 | |||||
| 7.75 | 100 | 88 | |||||
| 3.88 | 90.4 | 85 | |||||
| 1.9 | 42.2 | 54.1 | |||||
| 23-Y | 15 | 0 | 0 | Non-toxic | Non-toxic | ND | ND |
| 24-II | 15 | 100 | 100 | Toxic | Toxic | ND | ND |
| 7.5 | 100 | 100 | |||||
| 3.75 | 100 | 98 | |||||
| 1.88 | 86.6 | 96.7 | |||||
| 0.94 | 62.7 | 96 | |||||
| 25-III | 16.5 | 0 | 0 | Non-toxic | Non-toxic | ND | ND |
| 26-IV | 15 | 0 | 0 | Non-toxic | Non-toxic | ND | ND |
| 27-(V) | 30 | 0 | 4.1 | Non-toxic | Non-toxic | ND | ND |
| 28-(X) | 15.5 | 0 | 0 | Non-toxic | Non-toxic | ND | ND |
| 29-XI | 17 | 0 | 7.3 | Non-toxic | Non-toxic | ND | ND |
| 30- XII | 15 | 0 | 5.6 | Non-toxic | Non-toxic | ND | ND |
| 31-XIII | 15 | 85 | 98.5 | Toxic | Toxic | 5.6 | ND |
| 7.5 | 82.7 | 97.9 | |||||
| 3.75 | 76.8 | 75 | |||||
| 1.88 | 25.2 | 75 | |||||
| 0.94 | 4.7 | 0 | |||||
| 34-34 | 7.5 | 100 | 95.4 | Toxic | Toxic | 3.5 | 4.8 |
| 3.75 | 74.5 | 23.3 | |||||
| 1.88 | 31 | 0 | |||||
| 0.94 | 0 | 0 | |||||
| 0.47 | 0 | 0 | |||||
| 35-SB | 15 | 36 | 94.8 | Reduced-toxicity | Toxic | ND | 7 |
| 7.5 | 4.5 | 62.2 | |||||
| 3.75 | 0 | 37.6 | |||||
| 1.88 | 0 | 17.3 | |||||
| 0.94 | 0 | 7.8 | |||||
| 36-36 | 15 | 100 | 96.7 | Toxic | Toxic | 5.7 | 5.4 |
| 7.5 | 84.1 | 96.3 | |||||
| 3.75 | 71.4 | 52.3 | |||||
| 1.88 | 6.9 | 23.9 | |||||
| 0.94 | 0 | 7.5 | |||||
| 37-37 | 15 | 100 | 97.2 | Toxic | Toxic | 5.5 | 3.8 |
| 7.5 | 82 | 96.9 | |||||
| 3.75 | 79 | 92.1 | |||||
| 1.88 | 8.3 | 49.7 | |||||
| 0.94 | 2.4 | 2.3 | |||||
| 38-38 | 8.5 | 14.6 | 91.4 | Reduced-toxicity | Toxic | ND | 3.8 |
| 4.25 | 8.1 | 60.1 | |||||
| 2.125 | 3.2 | 43.3 | |||||
| 1.06 | 0.4 | 32.5 | |||||
| 0.35 | 0 | 8.7 | |||||
| 39-39 | 5 | 51.5 | 14.3 | Toxic | Reduced-toxicity | 4.9 | ND |
| 2.5 | 23.7 | 8.9 | |||||
| 1.25 | 8.1 | 2.7 | |||||
| 0.625 | 1.5 | 0.1 | |||||
| 0.313 | 0.27 | 0.3 | |||||
| 40-40 | 15.5 | 80.8 | 32.5 | Toxic | Reduced-toxicity | 10.3 | ND |
| 7.75 | 46.2 | 15.9 | |||||
| 3.875 | 3 | 8.7 | |||||
| 1.94 | 0 | 4.21 | |||||
| 0.97 | 0 | 0.3 | |||||
| 41-41 | 19.5 | 23 | 81.7 | Reduced-toxicity | Toxic | ND | 11.6 |
| 9.75 | 9.6 | 42.6 | |||||
| 4.88 | 1.2 | 24.3 | |||||
| 2.44 | 0.2 | 16.9 | |||||
| 1.22 | 0 | 0 | |||||
| 42-42 | 15.5 | 20.4 | 81.9 | Reduced-toxicity | Toxic | ND | 7.9 |
| 7.75 | 4.3 | 73 1 | |||||
| 3.875 | 0 | 32.9 | |||||
| 1.94 | 0 | 16.1 | |||||
| 0.97 | 0 | 0.1 | |||||
| 43-43 | 7.5 | 16.8 | 98.45 | Reduced-toxicity | Toxic | ND | 2.4 |
| 3.75 | 3.6 | 94.2 | |||||
| 1.88 | 0 | 60.5 | |||||
| 0.94 | 0 | 32.9 | |||||
| 0.47 | 0 | 15.2 | |||||
| 44-44 | 5 | 32.9 | 94.8 | Reduced-toxicity | Toxic | ND | 2.1 |
| 2.5 | 14.8 | 67 | |||||
| 1.25 | 6 | 47.1 | |||||
| 0.625 | 3.6 | 23.4 | |||||
| 0.313 | 0 | 8.1 | |||||
| 48-48 | 15.5 | 18.7 | 60.5 | Reduced-toxicity | Toxic | ND | 10.3 |
| 7.75 | 7.9 | 51.5 | |||||
| 3.875 | 0 | 42 | |||||
| 1.94 | 0 | 20.4 | |||||
| 0.97 | 0 | 4.2 | |||||
| 49-49 | 5.5 | 27.8 | 19.5 | Reduced-toxicity | Reduced-toxicity | ND | ND |
| 52-ML1 | 15 | 96.38 | 95.69 | Toxic | Toxic | 8.5 | 7.8 |
| 7.5 | 47.78 | 46.58 | |||||
| 3.75 | 9.84 | 28.49 | |||||
| 1.88 | 1.31 | 8.45 | |||||
| 53-ML2 | 16.3 | 58.01 | 60.80 | Toxic | Toxic | 14.4 | 13.8 |
| 8.15 | 24.97 | 25.04 | |||||
| 4.08 | 5.36 | 8.89 | |||||
| 2.04 | 1.31 | 1.56 | |||||
| 54-MF1 | 16.7 | 52.47 | 43.35 | Toxic | Reduced-toxicity | 17.4 | ND |
| 8.35 | 4.29 | 8.89 | |||||
| 4.18 | 0.67 | 0.27 | |||||
| 2.09 | 0.46 | 0 | |||||
| 55-MF2 | 8.25 | 38.19 | 36.03 | Reduced-toxicity | Reduced-toxicity | ND | ND |
| 56-2a | 15.55 | 50.55 | 51.97 | Toxic | Toxic | 16.4 | 15.1 |
| 7.78 | 8.34 | 8.89 | |||||
| 3.89 | 0 | 0 | |||||
| 1.9 | 0.88 | 0 | |||||
| Control 1a | 17 | 0 | 1.2 | Non-toxic | Non-toxic | ND | ND |
| Control 2a | 31 | 0 | 6.9 | Non-toxic | Non-toxic | ND | ND |
Cytotoxicity of Endophytic Fungal Crude Extractsa Against Normal (MRC-5) and Cancer (MCF-7) Cell Lines
Many endophytes are reported as a source for cytotoxic compounds, which were active against different cancer cell lines (20). Paclitaxel, camptotecin, podophyllotoxin, vinblastine, and vincristine were detected in endophytes (21). Minarni et al., found that ethyl acetate extracts of some endophytic fungi isolated from soursop leaf (Annona muricata L.) had a significant cytotoxic activity against MCF 7 cells (22). Also, cytotoxicity of endophytic fungi associated with Bostrychia tenella was evaluated against HL-60 (human leukemia), HCT-8 (human colon carcinoma), and SF-295 (glioblastoma); three samples exhibited efficient cell growth inhibition (80% - 100%) in all tested tumor cell lines (21).
Although it is assumed that the bioactive compounds of endophytes may possess a reduced cytotoxicity towards the eukaryotic cells not to harm their host plants found in a symbiotic relationship with them (2, 14), it is possible that these compounds do not harm the plant host since the plant may produce the same or similar compounds and, therefore, is tolerant to them (14) or these compounds may affect specific targets in the mammalian cells.
4.1. Conclusions
Endophytic fungi gained a great interest in the last decades since they represent a highly diversified group with promising biological activities. In the present study, endophytic fungi were isolated from medicinal plants inhabiting different geographical habitats to isolate diversified endophytic group with variable biological activities to produce promising bioactive compounds. Actually, some promising isolates were obtained where some isolates (i.e., Penicillium miczynskii 17-S and Penicillium miczynskii 25-III) showed antibacterial activities against both Gram-positive and negative strains and had no cytotoxicity against the tested cell lines to represent a good source for antimicrobial compounds safe for mammalian cells. Other isolates (i.e., A. terreus 35-SB, Nigrospora sp. 38-38, Penicillium sp. 41-41, Penicillium sp. 42-42, Cladosporium sp. 43-43, Aspergillus sp. 44-44, and Curvularia pallescens 48-48) exerted a toxic effect against the cancer cell line (MCF-7), but showed reduced toxicity against the tested normal cell line (MRC-5) to represent another good source for anticancer compounds with low toxicity against the normal cells.