1. Background
Opportunistic fungal infections are common in immunocompromised patients, which causes resistant fungi to antifungal agents (1). Oral candidiasis is a frequent infection in the oral cavity caused by different Candida species. According to the studies performed during the past two decades, Candida albicans, C. glabrata, and C. krusei are the most frequent pathogenic Candida species in the development of oral infections (2).
Azole drugs, as an important class of antifungals, affect the lanosterol 14α-demethylase, resulting in the inhibition of biosynthesis of the ergosterol in the fungal plasma membrane. These drugs contain fungistatic properties, and some of them, in particular fluconazole, can cause side effects in patients receiving azoles for a long time (3). In this regard, C. glabrata and C. krusei become quickly resistant to fluconazole. For this reason, finding novel and natural antifungal substances for clinical applications is necessary (4, 5). Thymol and carvacrol are phytocomponents classified as monoterpenes. They are the major components found in thyme (Thymus vulgaris) essential oil (6).
2. Objectives
The current study aimed to assess the anti-adherence and antifungal activities of thymol and carvacrol against C. albicans, C. glabrata, and C. krusei isolates obtained from patients with oral candidiasis concerning growth inhibition and fungal death as compared to the synthetic antifungals such as fluconazole and voriconazole.
3. Methods
3.1. Chemicals
The 1 µg disks of voriconazole and 25 µg fluconazole were purchased from Neo-sensitabs, Rosco Diagnostica, Denmark.
3.2. Isolation of Thymol and Carvacrol
Thymol and carvacrol were isolated from essential oils of Trachyspermum ammi and Zataria multiflora, respectively (7, 8). Thymol and carvacrol isolation was performed based on the column chromatography method. Briefly, 4 mL of essential oil was used for a silica gel column (1.5 cm i.d. and 22 cm in length). N-Hexane and ethyl acetate were used as elution solvents. The eluted fractions consisted of hydrocarbon constituents of essential oil in n-hexane (fraction A) and oxygenated constituents in ethyl acetate (fraction B). Carvacrol and thymol were purified from fraction B by preparative thin-layer chromatography (TLC). Toluene/ethyl acetate was used as a solvent system in preparative TLC. The purity of isolated carvacrol and thymol was validated by Gas chromatography-mass spectrometry (GC-MS). Stock solutions of thymol and carvacrol were prepared by 70°GL ethanol diluent.
3.3. Organism Identification
Clinical isolates of C. albicans (Ca1-10), C. glabrata (Cg1-10), and C. krusei (Ck1-10) were obtained from patients with oral candidiasis. Written informed consent was obtained from all participants. The identification of Candida strains was performed at the Mycology Research Center affiliated to the University of Tehran. The yeasts were grown on Sabouraud glucose agar (Merck Co., Darmstadt, Germany) and CHROM agar (CHROMagar Company, Paris, France) at 35°C for 48 h. The identification of isolated Candida strains was confirmed using the RAPID yeast plus system (Remel Inc., USA), as recommended by the manufacturer.
3.4. Disk Diffusion Method
Assessment of antifungal activity was performed using the disk diffusion method based on the CLSI-M44-A2 standard for yeasts (9). In brief, agar plates (90-mm diameter) containing Mueller-Hinton agar (Merck Co., Darmstadt, Germany) accompanied with glucose (2%), and methylene blue (0.5 mg) were used. Sterile cotton swabs dipped in yeast suspensions adjusted to 1 × 106 cells/mL were inoculated on the agar surface. Thymol, carvacrol (20 µL of stock solutions with 80 mg/mL concentration), fluconazole, and voriconazole disks were placed on the agar surfaces. Subsequently, the media was kept at 35°C and read at 24 h. After the colonies grew, the zones of inhibition around the disks were measured and recorded. All experiments were performed in duplicate. The interpretation of antifungal tests of standard drugs was done based on the CLSI protocol: zone diameters of ≥ 19 mm for fluconazole and ≥ 17 mm for voriconazole as susceptible (S); zone diameters of 15 to 18 mm for fluconazole and 14 to 16 mm for voriconazole as susceptible dose-dependent (SDD); and zone diameters of ≤ 14 mm for fluconazole and ≤ 13 mm for voriconazole as resistant (R).
3.5. Anti-adherence Assay
The yeasts inocula were adjusted in normal saline to 2 × 106 cells/mL. Stock solutions of treatments were prepared using 70°GL ethanol. The working solutions were prepared by diluting the stock solutions to two-fold sub-MIC concentrations of treatments using RPMI 1640 medium. Twofold dilutions of thymol, carvacrol, fluconazole, and voriconazole were dispensed into the 96-well flat-bottomed polystyrene microtiter plates in 100 µl volumes for each well. The inoculation was done using 100 µL of Candida suspension with the final concentration of 1 × 106 cells/mL. Wells without treatments (thymol, carvacrol, and antifungal drugs) were considered as positive controls. The inoculated media was kept at 35°C for 90 min with a shaking rate of 75 rpm. Then, the wells were washed three times with PBS to remove any residual planktonic cells, and stained with 1% solution (w/v) of crystal violet for 5 min. The optical densities (OD) for each well were determined at 540 nm wavelength using an ELISA reader. The relative adherence was calculated by dividing the OD value of a treated well by the positive control. To count the yeasts, the medium content was aspirated and air-dried. Then, it was fixed and gram stained (10).
3.6. Statistical Analysis
Data were subjected to analysis of variance (ONE-WAY ANOVA) and Tukey’s tests (SPSS software package, V. 17.0 for Windows, USA). Statistical significance was considered when P-value < 0.05.
4. Results
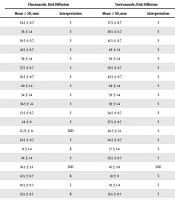
The results of inhibition zones of fluconazole and voriconazole against different Candida isolates, based on disk diffusion test, are shown in Table 1. Fluconazole susceptibilities were calculated as 100% for C. albicans, 50% for C. glabrata, and 0% for C. krusei strains. C. glabrata (20% SDD and 30% R) and C. krusei (50% SDD and 50% R) strains presented low susceptibilities to fluconazole. For voriconazole, 100% of C. albicans and C. krusei isolates and 90% of C. glabrata isolates were susceptible. As shown in Table 2, the mean diameter of the inhibition zone was greater for thymol than carvacrol in C. albicans (19.89 ± 0.80 mm versus 17.05 ± 0.61 mm), C. glabrata (18.87 ± 0.71 mm versus 15.77 ± 0.57 mm), and C. krusei (15.11 ± 0.91 mm versus 13.91 ± 1.04 mm) isolates tested. No significant difference was found between antifungal activities of thymol and carvacrol against various Candida species (P > 0.05).
| Candida albicans | Fluconazole, Disk Diffusion | Voriconazole, Disk Diffusion | ||
|---|---|---|---|---|
| Mean ± SD, mm | Interpretation | Mean ± SD, mm | Interpretation | |
| Ca1 | 34.5 ± 0.7 | S | 37.5 ± 0.7 | S |
| Ca2 | 36 ± 1.4 | S | 38.5 ± 0.7 | S |
| Ca3 | 39.5 ± 0.7 | S | 41.5 ± 0.7 | S |
| Ca4 | 41.5 ± 0.7 | S | 49 ± 1.4 | S |
| Ca5 | 36 ± 1.4 | S | 39 ± 1.4 | S |
| Ca6 | 37.5 ± 0.7 | S | 39.5 ± 0.7 | S |
| Ca7 | 38.5 ± 0.7 | S | 45.5 ± 0.7 | S |
| Ca8 | 40 ± 1.4 | S | 49 ± 1.4 | S |
| Ca9 | 34 ± 1.4 | S | 39 ± 1.4 | S |
| Ca10 | 36.5 ± 2.1 | S | 39 ± 1.4 | S |
| Cg1 | 33.5 ± 0.7 | S | 34.5 ± 0.7 | S |
| Cg2 | 24 ± 0 | S | 27.5 ± 0.7 | S |
| Cg3 | 15.75 ± 1.1 | SDD | 20.5 ± 2.1 | S |
| Cg4 | 22.5 ± 0.7 | S | 24.5 ± 0.7 | S |
| Cg5 | 11 ± 1.4 | R | 17 ± 1.4 | S |
| Cg6 | 24 ± 1.4 | S | 25.5 ± 0.7 | S |
| Cg7 | 16.5 ± 2.1 | SDD | 16 ± 2.8 | SDD |
| Cg8 | 12.5 ± 0.7 | R | 18 ± 0 | S |
| Cg9 | 18.5 ± 0.7 | S | 19 ± 1.4 | S |
| Cg10 | 13.5 ± 0.7 | R | 21.5 ± 0.7 | S |
| Ck1 | 33.5 ± 0.7 | S | 29 ± 1.4 | S |
| Ck2 | 24 ± 0 | S | 26 ± 1.4 | S |
| Ck3 | 15.75 ± 1.1 | SDD | 26 ± 1.4 | S |
| Ck4 | 22.5 ± 0.7 | S | 27 ± 1.4 | S |
| Ck5 | 11 ± 1.4 | R | 26.5 ± 0.7 | S |
| Ck6 | 24 ± 1.4 | S | 28 ± 0 | S |
| Ck7 | 16.5 ± 2.1 | SDD | 27.5 ± 0.7 | S |
| Ck8 | 12.5 ± 0.7 | R | 28.5 ± 2.1 | S |
| Ck9 | 18.5 ± 0.7 | S | 30.5 ± 0.7 | S |
| Ck10 | 13.5 ± 0.7 | R | 27.5 ± 0.7 | S |
Antifungal Susceptibility of Fluconazole and Voriconazole Against Candida albicans, Candida glabrata, and Candida krusei Isolated From Oral Candidiasis
| Candida Species | Thymol, Disk Diffusion, Mean ± SD, mm | Carvacrol, Disk Diffusion, Mean ± SD, mm |
|---|---|---|
| Ca1 | 19.85 ± 0.4 | 16.45 ± 0.4 |
| Ca2 | 18.75 ± 0.4 | 17.15 ± 0.4 |
| Ca3 | 20.15 ± 1.2 | 16.35 ± 0.4 |
| Ca4 | 20.25 ± 1.1 | 16.6 ± 0.4 |
| Ca5 | 19.4 ± 0.3 | 17.9 ± 0.1 |
| Ca6 | 20.6 ± 0.8 | 17.8 ± 0.4 |
| Ca7 | 19.05 ± 0.2 | 17.7 ± 0.1 |
| Ca8 | 19.8 ± 0.3 | 16.75 ± 0.4 |
| Ca9 | 20.9 ± 0.1 | 17.2 ± 0.3 |
| Ca10 | 20.2 ± 0.6 | 16.6 ± 0.3 |
| Cg1 | 19 ± 0.3 | 16.2 ± 0.3 |
| Cg2 | 18.5 ± 0.4 | 16.05 ± 0.2 |
| Cg3 | 19 ± 0.8 | 16.8 ± 0.3 |
| Cg4 | 20.05 ± 0.4 | 15.3 ± 0.3 |
| Cg5 | 19.35 ± 0.5 | 15.8 ± 0.1 |
| Cg6 | 18.75 ± 0.5 | 15.15 ± 0.2 |
| Cg7 | 17.8 ± 0.3 | 15.35 ± 0.5 |
| Cg8 | 18.5 ± 0.3 | 15.05 ± 0.2 |
| Cg9 | 19.4 ± 0.6 | 16.05 ± 0.1 |
| Cg10 | 18.35 ± 0.4 | 15.95 ± 0.1 |
| Ck1 | 16.2 ± 0.3 | 13.6 ± 0.3 |
| Ck2 | 16.05 ± 0.2 | 14.9 ± 0.4 |
| Ck3 | 16.8 ± 0.3 | 15.25 ± 0.4 |
| Ck4 | 15.3 ± 0.3 | 13 ± 0.1 |
| Ck5 | 15.8 ± 0.1 | 13.25 ± 0.2 |
| Ck6 | 15.15 ± 0.2 | 14.4 ± 0.4 |
| Ck7 | 15.35 ± 0.5 | 14.75 ± 0.1 |
| Ck8 | 15.05 ± 0.2 | 13.25 ± 0.2 |
| Ck9 | 16.05 ± 0.1 | 14.7 ± 0.4 |
| Ck10 | 15.95 ± 0.1 | 12 ± 0.1 |
Antifungal Susceptibility of Thymol and Carvacrol Against Different Pathogenic Candida Strains Isolated From Oral Candidiasis
As shown in Table 3, the results revealed that the lowest relative adherence ratio was among C. albicans isolates in comparison to other species. The mean relative adherence ratios (mean of adherence values of all treatments) for C. albicans, C. glabrata, and C. krusei were 0.50, 0.60, and 0.64, respectively. However, considering the absolute number of yeasts adhered to the polystyrene field, C. krusei was found to be adhered to the polystyrene surface with the lowest number of cells in comparison to the other two species. The mean absolute number of adherent C. albicans, C. glabrata, and C. krusei cells on the surface of the non-treated wells were 112.6 ± 16, 337 ± 45.9, and 73 ± 10.8 yeasts/field, respectively. Significantly higher numbers of C. glabrata cells could adhere to the polystyrene in comparison to C. albicans cells (P < 0.01, Tukey’s HSD test), but the difference between C. krusei and C. albicans was not significant (P > 0.05, Tukey’s HSD test), although a markedly lower number of C. krusei cells exhibited the ability to adhere. The most effective treatment to inhibit adherence among all species was thymol at which with the doses of 24.5, 25.5, and 35 µg/mL, it could inhibit the adherence of 72%, 67%, and 55% of C. albicans, C. glabrata, and C. krusei cells, respectively. Fluconazole was the least effective agent in preventing adherence for all species. However, voriconazole could inhibit the adherence of all three species more effectively than fluconazole. Also, thymol was more effective in preventing the adherence of yeast cells to polystyrene in comparison to carvacrol.
| Species | Antifungal Agents | |||||||
|---|---|---|---|---|---|---|---|---|
| Fluconazole | Voriconazole | Thymol | Carvacrol | |||||
| 1/2 MIC | 1/4 MIC | 1/2 MIC | 1/4 MIC | 1/2 MIC | 1/4 MIC | 1/2 MIC | 1/4 MIC | |
| C. albicans | 0.67 ± 0.09* | 0.78 ± 0.12 | 0.46 ± 0.09† | 0.55 ± 0.08† | 0.28 ± 0.07† | 0.4 ± 0.06† | 0.41 ± 0.06† | 0.5 ± 0.09† |
| C. glabrata | 0.75 ± 0.12 | 0.83 ± 0.12 | 0.64 ± 0.06† | 0.76 ± 0.1 | 0.33 ± 0.04† | 0.42 ± 0.07† | 0.45 ± 0.06† | 0.52 ± 0.07† |
| C. krusei | 0.86 ± 0.14 | 0.93 ± 0.07 | 0.5 ± 0.05† | 0.58 ± 0.07† | 0.45 ± 0.07† | 0.52 ± 0.07† | 0.6 ± 0.07† | 0.65 ± 0.06† |
The Relative Adherence (Mean ± Standard Deviation) of C. albicans, C. glabrata, and C. krusei After Treatment with Azole Drugs, Thymol, and Carvacrol Quantified by Crystal Violet Staininga
5. Discussion
The high occurrence of oral candidiasis in patients with underlying disorders has encouraged scientists to search for novel and natural antifungal agents (4). In this study, 50% of C. krusei strains were resistant to fluconazole, according to the results of the disc diffusion susceptibility testing method. This finding is not consistent with some studies that reported resistance rates higher than 80% (2). In a worldwide study by Pfaller et al. (11), the resistance of C. glabrata strains to fluconazole is reported as 5.9% in Brazil, 36% in Venezuela, 21.7% in Ecuador, 18.2% in Colombia, and 14.6% in Argentina. In our experiments, although voriconazole was highly active against C. glabrata, susceptible dose-dependent isolates to voriconazole were also detected, as confirmed by the worldwide data reported (2). The discrepancy of C. glabrata susceptibility to azoles could be related to long-term use of fluconazole and/or itraconazole in order to treat infections caused by Candida species (12).
In this study, both thymol and carvacrol revealed remarkable effects against Candida species, in particular the resistant strains. Disk diffusion assay has been performed on fluconazole-resistant Candida species, such as C. glabrata and C. krusei. Moreover, these species also demonstrated susceptibility to 80 mg/mL concentration of thymol and carvacrol. Previous studies reported the potent anti-Candida activity of thymol and carvacrol (13, 14). In agreement with our results, other scholars reported higher antifungal activity of thymol than carvacrol (15). The presence of a phenolic hydroxyl at different locations on the phenolic ring of thymol (C3) and carvacrol (C2) influences the degree of antifungal activity (16-18). Consistent with the findings of the present study, Ahmad et al. (16) demonstrated that thymol (100 µg/mL) and carvacrol (75 µg/mL) had fungicidal activity against fluconazole-susceptible and -resistant Candida species. In fact, the monoterpenes, such as thymol and carvacrol, were associated with decreased ergosterol content of the fluconazole- susceptible and -resistant Candida strains (16).
In this study, the most effective treatment to inhibit adherence among all species was thymol. So that with the doses of 24.5, 25.5, and 35 µg/mL, it could inhibit the adherence of 72%, 67%, and 55% of C. albicans, C. glabrata, and C. krusei cells, respectively. Fluconazole was the least effective treatment in preventing adherence for all species. However, voriconazole could inhibit the adherence of all three species more effectively than fluconazole. Samaranayake et al. (19) indicated an association between hydrophobicity and adherence of C. albicans and C. krusei to the HeLa cells. In another research, C. glabrata showed a four-fold higher relative cell surface hydrophobicity (CSH) and a two-fold higher tendency to attach on the acrylic surfaces compared to C. albicans, at which highly a significant correlation was found between hydrophobicity and adherence (20). However, some Candida species with high adherence profiles also showed good biofilm mass formation (21). There are frequent reports on the superior adherence ability of non-albicans Candida species to host cells and synthetic surfaces in comparison to C. albicans (19). Voriconazole (at the doses of 0.06 - 16 µg/L) showed significant anti-biofilm activity against C. albicans, C. glabrata, and some other Candida spp. The reduction rates for C. albicans and C. glabrata were 64.5 and 23.8%, respectively, indicating more resistance of C. glabrata. The authors explained that the inhibition may be due to yeast cell surface modifications and interruption of the adhesion process in biofilm formation (22). In the present study, although voriconazole showed promising anti-adhesion activity against all evaluated species but fluconazole was not as effective as voriconazole. Our results are consistent with other studies indicating more resistance of non-C. albicans species against the anti-adhesion effect of azole drugs, particularly fluconazole. Thymol and carvacrol have been shown to be potent biofilm inhibitors, at which thymol showed significant anti-biofilm activity at half of the dose required by carvacrol (23). Moreover, thymol demonstrated greater anti-biofilm and anti-cell surface hydrophobicity activity than that by fluconazole against C. albicans (24, 25). Interestingly, the anti-adhesion activity of these natural compounds has been established at sub-MIC doses indicating a possible specific anti-adherence activity regardless of their fungicidal effect. This assumption is in accordance with the results of other studies indicating the potent specific anti-biofilm/anti-adherence effect of some phenolic terpenoids (23).
5.1. Conclusions
In summary, this study demonstrated that voriconazole had higher activity than fluconazole against various Candida spp tested, in particular resistant strains. Thymol and carvacrol exhibited fungicidal activity against clinical isolates of C. albicans, C. glabrata, and C. krusei strains. Also, thymol was more effective in the case of preventing the adherence of yeast cells to polystyrene in comparison to carvacrol.