1. Background
Oxidative stress is a basic principle of most diseases and has an important role in the source and growth of many illnesses such as cardiovascular disease and cancer (1). In order to detect new compounds with higher antioxidant activity, natural compounds segregated of plants are good candidates to study their antioxidant and other biological activities (2). ScrophulariaL. is the largest genus of Scrophulariaceae in Iran (3, 4). Scrophularia umbrosa Boiss (S. umbrosa), with common name “water figwort”, is one of the native species of Iran (5). The scientific name of Scrophularia is derived from traditional use of this plant as a remedy of scrofula (6). Several species of the Scrophularia have biologically active phenyl ethanoids, phenyl propanoids, flavonoids, iridoids, iridoid glycosides, and terpenoids (7-10). Many Scrophularia species have been used in Asian countries as a medicinal herb for the treatment of various diseases such as allergy, rheumatics, and chronic inflammatory disorders. Furthermore, several species of Scrophularia have numerous biological activities such as antioxidant, antitumor, antiprotozoal, antibacterial, diuretic, and hepatoprotective properties. In addition, they have been used as a remedy to gastrointestinal, nervous, and mental disturbances (11-14).
2. Objectives
In continuation of our scientific works on the analysis of the volatile and non-volatile components of the Iranian medicinal herbs along with their biological activities, (15-17), now, we have studied the MeOH, DCM and n-Hexane extracts of aerial parts, and seed of S. umbrosa, which is commonly known as water figwort in Iran. As far as we know, no research has been conducted on pharmacological and biological activates or chemical composition of S. umbrosa. In this article we report the antimalarial activity and free radical scavenging of methanol, DCM and n-Hexane extract from aerial parts and seed of this plant, moreover total flavonoid and total phenol contents. The NMR technique was used to identify the ingredients of MeOH extract and GC-MS. In addition, the GC-FID techniques was used for the identification of components of DCM and n-Hexane extracts of aerial parts and seed from S. umbrosa.
3. Methods
3.1. Chemicals
Folin ciocaltea reagent and gallic acid were purchased from Fluka. DPPH was acquired from Sigma (Germany). All chemicals and solvents were analytical grade.
3.2. Plant Material, Extraction, and Fractionation
The aerial parts and seed of S. umbrosa were collected from Mishodaghi Mountain in East Azarbaijan province during the flowering period. The identification of the plant was approved by anatomical assessment compared with the herbarium sample (voucher Nos. Tbz-Fph-762) preserved in the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran.
Air-dried ground of aerial parts and seed (100 g) from S. umbrosa were extracted with n-hexane, dichloromethane (DCM), and methanol (MeOH) in Soxhlet apparatus, consecutively. Captured extracts were separately concentrated at a maximum temperature of 45°C by rotary evaporator. From the aerial parts, 3.45 g, 1.16 g, and 15.72 g were extracted and 32.68 g, 4.57 g, and 8 g from the seeds were gained.
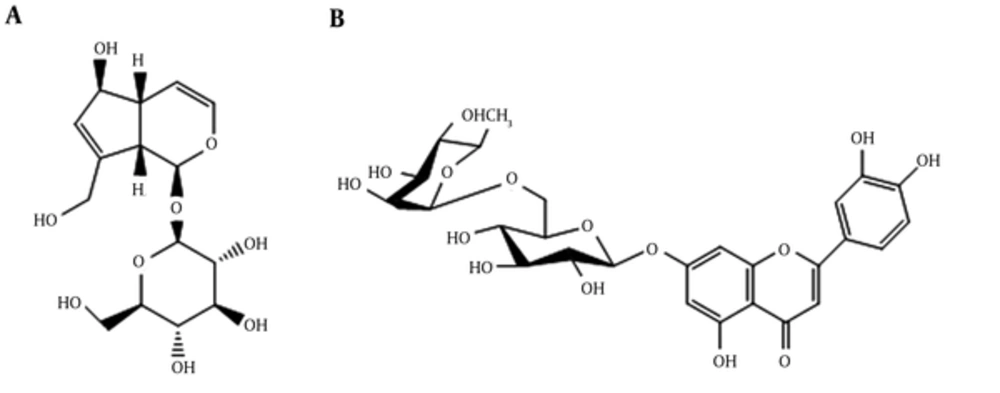
A total of 2 grams of the dried extract of MeOH was subjected to solid-phase extraction (SPE) on Sep-Pak (Vac 35 mL; 10 g; C18 cartridges, Waters, Ireland) by a series of MeOH, Water mixture (10/90, 20/80, 40/60, 60/40, 80/20, and 100/0) as eluent. Solvent of fractions were removed at a maximum temperature of 45°C by rotary evaporator. For isolating the phytochemicals is added. isolate phytochemials, the SPE fraction eluted with 10% MeOH was analyzed by reversed-phase preparative HPLC analysis (Knauer, Germany) using the mobile phase. Structural identification of all compounds were elucidated unequivocally by spectroscopic means and comparing with references. It allowed 1 iridoid and 1 flavonoid structures, which were identified unequivocally as aucubin (A) and Luteolin-7-o-rutinoside (B).
3.3. Free Radical Scavenging Assay
The potency of these extracts and fractions to suppression of free radicals was determined by the DPPH method (18-20). The percentage reduction was drawn against the sample extract concentration to calculate RC50 values (the concentration the extract that provide 50% loss of DPPH activity). Quercetine was used as a positive control. All tests were performed in duplicate.
3.4. Total Phenol Content (TPC)
Total contents of the phenolic compounds were detected by the modified Folin- Ciocalteau method (21). The calibration curve was drawn with Gallic acid. This test was performed twice for each sample.
3.5. Total Flavonoid Content (TFC)
Total flavonoid content of these samples was detected using a modified colorimetric assay (22). The standard was Rutin. The result was expressed as rutinoside equivalents in mg per 100g of dried extract.
3.6. GC-MS and GC-FID Analyses
DCM and n-Hexane extracts of aerial parts and seed from S. umbrosa were excavated using a Shimadzu GCMS-QP5050A gas chromatograph-mass spectrometer (GC-MS). Recognition of ingredients was based on direct comparison of the Kovats Indices (KI) and MS data with those listed in the Adams, the Wiley229, and NIST107 mass spectral libraries (23, 24). For quantitation (area%), the GC analysis was also performed on a Shimadzu GCMS-QP5050A gas chromatograph fitted with a FID detector.
3.7. Antimalarial Test
The potential antimalarial effect of this plant material was examined by the Afshar et al. method (25), with some changes. The results were reported as % inhibition (1%) of hem polymerization/crystallization compared to a positive control (chloroquine) by the following formula, 1% = ((AB - AA)/AB) × 100
AB, blank absorbance.
AA, test samples absorbance.
4. Results
A combination of solid phase extraction (SPE) and reversed-phase prep-HPLC analyses of the MeOH extract of the aerial parts and seed of S. umbrosa led to the characterization of 1 known iridoid glycosides aucubin (A) (26) and 1 known flavonoid Luteolin-7-o-rutinoside (B) (27, 28) (Figure 1). All data were in accordance with the relative published data.
Aucubin (A), amorphous powder, 1HNMR (400MHz, D2O): δ 5 (br.s, 1H, H-1), 6.18 (d, 1H, J = 5.7, H-3), 5.18 (d, 1H, J = 4.5Hz, H-4), 2.68 (br.s, 1H, H-5), 4.43 (brs, 1H, H-6), 5.74 (brs, 1H, H-7), 3.03 (br.s, 1H, H-9), 4.16 (d, 1H, J = 15 Hz, H-10a), 4.23 (d, 1H, J = 15 Hz, H-10b), 4.73 (1H, H-1’), 3.63 (br.d, 1H, H-6’a), 3.80 (br.d, 1H, H-6’b), 3.20- 3.42 (remaining sugar protons). 13CNMR (100MHz, D2O): 94.14 (C-1), 138.34 (C-3), 104.05 (C-4), 41.11 (C-5), 79.34 (C-6), 127.29 (C-7), 145.67 (C-8), 45.16 (C-9), 59.45 (C-10), 97.19 (C-1’), 71.57 (C-2’), 74.48 (C-3’), 68.38 (C-4’), 75.01 (C-5’), 58.29 (C-6’). Data were in agreement with the published data (26).
Luteolin-7-o-rutinoside (B), amorphous powder, 1HNMR (400 MHz, DMSO-d6): δ 6.91 (s, 1H, H-3), 6.45 (s, 1H, H-6), 6.74 (s, 1H, H-8), 7.41 (1H, H-2’), 6.91 (br.d, J = 6.22, 1H, H-5’), 7.44 (d, J = 8.17, 1H, H-6’), 12.99 (s, 1H, OH), 5.07 (d, J = 7.03, 1H, H-1”), 4.5 (br.d, 1H, H-1”’), 1.06 (d, J=6.04, 3H, H-6”’), 3.11-3.84 (remaining sugar protons). Data were in agreement with the published data (27, 28).
The results of free radical scavenging activity, total phenolic, and total flavonoid contents were obtained from extracts and its fractions of S. umbrosa are given in Table 1.
| Extracts and Fractions | Total Phenol Content (TPC), mg/100g | Total Flavonoid Content (TFC), mg/100g | Antioxidant Activity (RC50), mg/L | |||
|---|---|---|---|---|---|---|
| Aerial Part | Seed | Aerial Part | Seed | Aerial Part | Seed | |
| n-Hexane | 4.84 ± 0.693 | 4.00 ± 0.000 | 10.140 ± 0.195 | 6.410 ± 0.265 | 0.258 ± 0.002 | 5.528 ± 3.123 |
| DCM | 5.75 ± 1.287 | 4.00 ± 0.000 | 17.253 ± 0.016 | 7.010 ± 0.366 | 1.27 ± 0.741 | 1.571 ± 0.172 |
| MeOH Extract | 7.01 ± 1.782 | 5.05 ± 0.792 | 9.076 ± 0.156 | 16.08524 ± 0.125 | 0.152 ± 0.048 | 0.272 ± 0.046 |
| 10% MeOH-water fraction | 4.70 ± 0.099 | 4.00 ± 0.000 | 0.872 ± 0.008 | 1.22477 ± 0.039 | 1.458 ± 0.209 | 1.676 ± 0.836 |
| 20% MeOH-water fraction | 11.35 ± 0.198 | 9.46 ± 0.693 | 14.085 ± 0.2571 | 2.05678 ± 0.000 | 0.056 ± 0.004 | 0.059 ± 0.013 |
| 40% MeOH-water fraction | 18.91 ± 2.673 | 23.25 ± 2.079 | 32.428 ± 0.078 | 33.7503 ± 0.078 | 0.032 ± 0.003 | 0.016 ± 0.0003 |
| 60% MeOH-water fraction | 10.72 ± 1.584 | 8.13 ± 0.792 | 10.950 ± 0.000 | 10.72401 ± 0.086 | 0.098 ± 0.019 | 0.076 ± 0.003 |
| 80% MeOH-water fraction | 6.87 ± 0.099 | 6.87 ± 0.198 | 12.212 ± 0.008 | 2.89981 ± 0.179 | 0.281 ± 0.006 | 0.107 ± 0.036 |
| 100% MeOH-water fraction | 6.80 ± 1.980 | 4.42 ± 0.099 | 3.098 ± 0.039 | 8.28308 ± 0.530 | 0.507 ± 0.290 | 0.301 ± 0.017 |
| Quercetine | - | 0.005 ± 0.0009 | ||||
| Rutin | - | 0.00975 ± 7.07E-05 | ||||
Total Phenol Content (TPC), Total Flavonoid Content (TFC), and Antioxidant Activity of the DCM, n-Hexane, and Methanolic Extract and Fractions of Aerial Parts and Seed of S. umbrosa
In this study, total extracts of the aerial parts and seed were evaluated to antimalarial activity using the assay of In vitro β-hematin formation. The n-hexane and MeOH extracts of aerial parts and seed did not exhibit any significant inhibition of heme bio crystallization properties. However, the DCM extracts of aerial parts, in comparison with the blank, exhibited partly antimalarial effects (Figure 1). In less concentrations (0.1 - 0.4 mg/mL) and (0.4 - 2 mg/mL) the revealed absorbance of the DCM, extracts were higher than the blank. It might be the outcome of other fatty acids present in extracts that cause a synergistic effect with oleic acid in the experiment. At higher concentrations (> 2 mg/mL), the DCM extracts prevented heme bio crystallization. At higher concentrations (> 2 mg/mL), the DCM extracts showed powerful antimalarial effects. The IC50 value of the DCM extracts (aerial parts) was 2.67 mg/mL compared with chloroquine 0.014 mg/mL as a positive control.
Recognition of the DCM and n-Hexane extracts of various organs of S. umbrosa compounds was based on the straight comparison of the Kovats Indices (KI) and MS data with those for standard alkanes, and computer matching with those listed in the Adams, the Wiley229, and NIST107 mass spectral libraries and also published data (10, 29, 30), which is listed in Table 2.
| Retention Time, min | Compounds | K. I. | % | |
|---|---|---|---|---|
| DCM extract of aerial parts | ||||
| 1 | 27.844 | Neophytadiene | 1815 | 7.85 |
| 2 | 29.977 | Hexadecanoic acid | 1915 | 9.83 |
| 3 | 33.386 | Methyl linolelaidate | 2077 | 54.90 |
| Total identified = 72.58 | ||||
| DCM extract of seed | ||||
| 1 | 29.373 | Methyl palmitate | 1900 | 1.50 |
| 2 | 30.011 | n-Hexadecanoic acid | 1915 | 7.82 |
| 3 | 33.477 | Methyl linolelaidate | 2077 | 70.15 |
| Total identified = 79.48 | ||||
| n-Hexane extract of aerial parts | ||||
| 1 | 33.297 | Phytol | 2043 | 3.12 |
| 2 | 43.491 | Heneicosane | 2100 | 4.45 |
| 3 | 45.11 | Tetracosane | 2400 | 78.56 |
| 4 | 50.822 | Tetratriacontane | 3400 | 7.58 |
| 5 | 51.41 | Vitamin E | - | 3.64 |
| Total identified = 97.36 | ||||
| n-Hexane extract of seed | ||||
| 1 | 30.003 | Palmitic acid | 1940 | 4.35 |
| 3 | 33.47 | Methyl linolelaidate | 2077 | 39.87 |
| 4 | 33.59 | 9,12-Octadecadienoic acid (Z,Z) | 2106 | 10.39 |
| 5 | 43.51 | Octacosane | 1800 | 2.69 |
| 6 | 46.845 | Nonacosane | 1900 | 11.57 |
| 7 | 49.6 | γ.-Tocopherol | - | 4.36 |
| 8 | 57.26 | γ-Sitosterol | 3220 | 19.81 |
| Total identified = 93.04 | ||||
GC-MS and GC-FID Data of the Components of the DCM and n-Hexane Extract of Aerial Parts and Seed of S. umbrosa
5. Discussion
The antioxidant assay of n-Hexane, DCM, MeOH extracts, as well as its fractions determined by the DPPH method is based on the ability of antioxidants to accept electron or hydrogen and to become a stable diamagnetic molecule (Diphenylpicrylhydrazyl). It was found that all extracts and fractions reduced DPPH radicals in a dose dependent manner. The lower RC50 values show a higher ability of the antioxidant to scavenge the DPPH radicals and the higher RC50 values indicates a lower scavenging activity of the scavengers. Compared to the standard antioxidant Quercetine (RC50 0.0039 ± 0.5 mg/mL) n-Hexane, DCM and MeOH extracts and its fractions exhibit weak to strong radical scavenging activities (RC50 0.016 - 5.528 mg/mL), however, the free radical scavenging of 40% MeOH-water fraction of seed (RC50 0.016 ± 0.0003) was higher than others.
The radical scavenging activity in the aerial part and seed extracts as well as fractions decreased in the following order:
Aerial parts, 40% > 20% > 60% > MeOH > n-Hexane > 80% > 100% > DCM > 10%
Seed, 40% > 20% > 60% > 80% > MeOH> 100% > DCM > 10% > n-Hexane
Used method in this experiment is based on the reduction of the DPPH by methanolic solution in the presence of a hydrogen-donating scavenger through formation of the non-radical DPPH (20). All experiment extracts were able to scavenge DPPH free radicals and showed a dose-dependent manner. The suppression activities of DPPH to some methanolic fractions were comparable by quercetin (RC50 values 0.016 ± 0.0003 mg/mL for 40% seed, 0.032 ± 0.003 mg/mL for 40% aerial part and 0.0039 ± 0.5 mg/mL for quercetin). MeOH extracts showed drastic activity than DCM and n-Hexane extracts. It seems that MeOH extracts may contain phenolic compounds, e.g. flavonoids, coumarins, or phenyl propanoids, which contribute in significant free radical scavenging activity of this extract. Phenolic content was specified in comparison with Gallic acid as standard (Table 1). According to our results, 40% MeOH-water fractions of seed and40%, 20%, 60% MeOH-water fractions of aerial parts contained more phenolic contents than the others. Total flavonoid content (TFC) was determined in comparison with standard. The TFC values for 40% MeOH-water fractions of seed and 40% MeOH-water fractions of aerial parts were 33.75 and 32.43 mg rutinoside per 100 g dry sample, respectively (Table 1). According to previous studies, there is a direct link between total phenolic contents and antioxidant activity of the plants (31-33). Flavonoids, flavonols, flavones, and condensed tannins, are a class of plant phenolic, which are responsible for the radical scavenging and chelating properties (34, 35).
According to our findings, total flavonoids and total phenol content of 20%, 40%, and 60% fractions of MeOH-water were more than the other fractions in parallel to the free radical scavenging of these fractions. Different types of phenolic compounds possess different antioxidant capacities, which are related to their chemical structure (32). The hydroxylation of ring B and a C2 - C3 double bond connected with a C3 hydroxyl group and a C4 carbonyl group are critical structural components of flavonoids to suppression of hydroxyl radicals. Hydroxylation of ring A augments this activity (1).
In result of ingestion and digestion of the hemoglobin from host cell, the erythrocytes that are infected by malaria are specified by eminent amount of ferri proto porphyrin IX (heme) production (35). The primary food source during intra erythrocyte development and proliferation to the parasite is hemoglobin (36). The released Heme in this process is a toxic material (37). The formation of gloomy micro crystals of hemozoin in the vacuoles of malaria parasites, commonly known as malaria pigment, is an essential mechanism of heme detoxification (38-40). It has been revealed that a cyclic dimer of ferriprotoporphyrin IX (heme) forms Hemozoin. Various surveys have revealed that inhibition of β-hematin formation under different conditions is performed by chloroquine and other antimalarial ingredients (40). These facts are the foundation of the heme bio crystallization and inhibition assay (41, 42).
GC-MS data showed that volatile portions of DCM and n-Hexane extracts of various organs from S. umbrosa are containing a few identifiable compounds as follow, much of the DCM extract of aerial parts (54.9%) is including methyl Linoleic acid. This compound also exists in the DCM extract of the seed (58.44%). In addition to the alkanes, n-Hexane extract of aerial parts have a few percent of vitamin E (3.64%) and phytol (3.1%). Furthermore, there is gama-citostrol (19.81%) in n-Hexane extract of seed.
The results of 1HNMR spectroscopy are parallel with findings that have been reported in Table 1. Fractions, which show peaks in aromatic regions at δH 6-8 pm, possess polyphenolic compounds and lower RC50 values. The 1HNMR spectera of 10% MeOH-water fractions belonging to S. umbrosa have revealed that there is no flavonoid compounds in this fraction, however, some other phenols may exist in low concentration in this fraction that cause antioxidant activity. Obviously the spectrums showed that flavonoid compounds exist in high concentrations in 40% and 60% MeOH-water fractions of this plant. Solid phase extraction of the polar extract from S. umbrosa pursued HPLC analysis of the MeOH extract of aerial parts (10% fraction) and MeOH extract of seed (40% fraction) led to identification of iridoid and flavonoid structures. Compounds 1 and 2 were identified as aucubin (A) and Luteolin-7-O-rutinoside (B) through straight analogy of spectroscopic results with data published in the literature (18-20, 43-46).
Also based on our results, it is concluded that a fractionation of extracts and running their 1HNMR could be a valuable method for predicting of natural compounds and interfacing of chemical and biological assessments.