1. Background
In recent years, many biological compounds have been obtained from various sea animals such as tunicates, sponges, soft corals, sea hares, nudibranchs, bryozoans, sea slugs (1). Some of these bioactive compounds are also involved in biological activities including reproduction, communication, and elimination of infection (2). On the other hand, marine animals with a soft body and dwelling habitat do not have the conventional defensive equipment, and usually use toxic compounds as chemical weapons and strong physiological inhibitors to survive (3).
Cnidarians are classified into four main classes: Hydrozoa, Scyphozoa, Cubozoa, and Anthozoa (4). Sea anemone is from the class Anthozoa and has a short cylindrical body. The oral disk (with a mouth in the middle), column and pedal disks are three main parts of sea anemones. The oral disk is a flat screen above the body (5). There are many explosive cells, named cnidocyts on the tentacles, around the mouth and even found sporadically in other parts of the body. Cnidocyt cells having a giant poisonous sub-cellular cell called a nematocyst that the organism fires when motivated (6). The proteins and peptides in these toxins are actually bioactive compounds that have properties like cytolysins (7), neurotoxins (8), protease inhibitors (9) and phospholipase A2 (10).
Breast cancer is the most common cancer as well as the first cause of death among women around the world. In addition, while 18% of the cancers in women are currently formed, its annual prevalence is increasing in the world (11). MCF-7 as the most common breast cancer cell line is very useful in basic and applied sciences for evaluating the effects of drugs in vitro on cancer cells (12).
Several studies have been performed on the effects of marine bioactive compounds on cancer cells and their cytotoxic effects. The effect of sea anemone toxin, including those of Bunodosoma caissarum, Heteractis magnifica and H. crispa on the cancer cell lines U87, A549, T47D and A431 (13, 14) has been previously studied. RTX-A extracted from H. crispa showed cytotoxic effects on cancer cells such as HL-60, MDA-MB-231, HELA, THP-1 and SNU-CU (15).
The particular conditions of the Persian Gulf as a closed ecosystem are a good opportunity to study biological and toxic activity in toxic animals. Hence, the present study aimed to investigate the inhibitory effects of crude extracts and proteins/peptides obtained from the oral disk of the sea anemone Stichodactyla haddoni on the breast cancer cell line MCF-7.
2. Methods
Samples of sea anemones were collected from the coast of Hormuz Island (27.0593° N, 56.4608° E) in the Persian Gulf at low tide. The oral disks of specimens (number 5) were placed in a nitrogen tank, transferred to the laboratory and kept at -70°C. Determination of the studied species was carried out with identification key (9).
2.1. Phosphate Buffered Saline (PBS) Extract
The oral disks were completely homogenized using a homogenizer with a ratio of 1:5 with a sterile phosphate buffered saline (PBS) (pH = 7.4). In the next step, the homogenized samples were placed in a shaker incubator at 4°C for 150 minutes at 90 rpm. The supernatant was then centrifuged at 10000 rpm at 4°C for 10 minutes in a microcentrifuge. After centrifugation, 3 phases were formed consisting of top-down: (1) white and creamy waxes, (2) orange-yellow clear liquid, and (3) cellular deposits. The second phase was used to evaluate the anticancer effects. Finally, the crude extracts were lyophilized with a freeze dryer. In addition, all extraction steps were performed on the ice at 4°C.
2.2. Total Protein Assay
The total protein content of the crude PBS extract was determined using the bicinchoninic acid (BCA) method (16). The protein content of the samples was measured using an Elisa Reader at 562 nm.
2.3. Ultrafiltration
The separation of various components of the PBS crude extract was performed with Millipore filters (Amicon, Franc) of 3, 10, 30 and 100 kDa. Fractions with molecular weights > 100 kDa (Fraction 1), 30 - 100 kD (Fraction 2), 10 - 30 kDa (Fraction 3), 3 - 10 kDa (Fraction 4), and < 3 kDa (Fraction 5) were obtained from the Millipore filters. All filters were used at 4°C and at 5000 rpm.
2.4. Cell Line and Cell Culture
MCF-7 (C135) was purchased from the Pasteur Institute of Iran (Tehran, Iran). Cell culture medium used was 1640 RPMI (Sigma Chemicals Co., USA) enriched with 10% bovine serum albumin (BSA) (Gibco, Invitrogen Corporation, USA) and 1% penicillin.
2.5. Cell Viability and Cytotoxicity Assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) test was used to evaluate cytotoxic effects (17). This test is based on the reduction and breakdown of tetrazolium yellow crystals. The quantity of formazan is measured by recording changes in absorbance at 570 nm using an Elisa Reader. Doxorubicin and PBS with pH 7.2 were used for positive and negative controls, respectively. In addition, the 10 wells containing only the cell and culture medium were considered as the base control.
The concentration of toxin that can inhibit 50% of cell division is shown by the kinetic component IC50 (half maximal inhibitory concentration).
2.6. Reversed-Phase HPLC Analysis
Protein and peptide fingerprints of S. haddoni were detected using reverse phase gradient HPLC (KNAUER) with UV detector (K2600) and C18 column (Eurosil Bioselecta 300-C, 250 × 4.6 mm). The samples (fractions 1 - 5) were directly used for HPLC injection. The injection volume was 20 μL and detection was carried out at 214 nm. The retention of a peptide expressed as percentage of acetonitrile (%ACN) was estimated from the following equation:
%ACNe = %ACN0 + (%ACN/tG) (tR - t0 - tD)
Where %ACN0 is percentage of acetonitrile at the gradient start time (10%), %ACN/tG: the gradient slope (50%/60 minutes = 0.85%/minutes), tR: the retention time of compound X, t0: the elution time of a non retained compound (2.8 minutes), tD: the equipment dwell time (0.1 minute).
2.7. Statistical Analysis
All data were expressed as mean ± SD. After determining the normality of the data by Kolmogorov-Smirnov test, one-way ANOVA and Duncan post hoc test were used to determine the significant difference between data obtained from the crude extract and their fractions with a confidence level of 95%. The comparison of parameters between the 24 and 48 hours exposures was done by the independent t-test.
3. Results
3.1. Total Protein
The total protein content of the crude extracts and fractions 1 - 5 are shown in Table 1. The highest total protein was measured in fraction 2.
| Sea Anemone Compounds, Concentration (µg/ml) | Cell Viability (24 Hours)b, % | Cell Viability (48 Hours)b, % | Concentration of Total Protein/Peptideb, µg/mL | IC50 (24 Hours), µg/mL | IC50 (48 Hours), µg/mL |
|---|---|---|---|---|---|
| Crude extract | 8231 ± 43 | 894 | 677 | ||
| 0 (control) | 100 ± 3.6A | 100 ± 1.8A | |||
| 200 | 100 ± 1.9A | 100 ± 3.1A | |||
| 400 | 65 ± 6.3B | 56.2 ± 4.7C | |||
| 600 | 42.4 ± 3.4D | 33.3 ± 5.4E | |||
| 800 | 32.7 ± 2.4E | 24.8 ± 4.1F | |||
| 1000c | 22.6 ± 0.6FG | 14 ± 3.0G | |||
| 1200c | 12.5 ± 1.4G | 8 ± 2.0G | |||
| 1400c | 12 ± 1.0G | 7.6 ± 3.4G | |||
| Fraction 1 | 1305 ± 27 | 204 | 246 | ||
| 0 (control) | 100 ± 2.3A | 100 ± 4.1A | |||
| 200 | 100 ± 4.7A | 100 ± 6.3A | |||
| 400 | 62 ± 1.9B | 53.6 ± 4.7C | |||
| 600c | 53.3 ± 3.4D | 42.3 ± 1.8E | |||
| 800c | 52.9 ± 2.5E | 42.1 ± 3.3F | |||
| 1000c | 53 ± 7.5F | 42.8 ± 3G | |||
| 1200c | 52 ± 3.1H | 41.9 ± 1.1H | |||
| 1400c | 51.8 ± 0.0.5H | 41.2 ± 1.7H | |||
| Fraction 2 | 2154 ± 15 | 667 | 625 | ||
| 0 (control) | 100 ± 2.2A | 100 ± 3.5A | |||
| 200 | 100 ± 1.5A | 100 ± 4.2A | |||
| 400 | 61.5 ± 4.2B | 52 ± 3.8C | |||
| 600 | 44.2 ± 5.3D | 35.2 ± 1.7E | |||
| 800c | 23.5 ± 2.8F | 14.2 ± 2.9G | |||
| 1000 | 8 ± 1.3H | 5.2 ± 2.2H | |||
| 1200 | 8.4 ± 0.4H | 6 ± 1.8H | |||
| 1400 | 7.8 ± 1.3H | 5 ± 1.8H | |||
| Fraction 3 | 1740 ± 11 | 882 | 136 | ||
| 0 (control) | 100 ± 2.4A | 100 ± 2.7A | |||
| 200 | 100 ± 3.3A | 100 ± 4.7A | |||
| 400 | 100 ± 1.5A | 100 ± 1.4A | |||
| 600c | 79.4 ± 5.7B | 70 ± 2.3DE | |||
| 800c | 77.9 ± 1.6BC | 69.3 ± 3.2E | |||
| 1000c | 74.3 ± 4.5C | 65.1 ± 4.6E | |||
| 1200c | 72.1 ± 2.45D | 64 ± 3.9E | |||
| 1400 | 71.5 ± 6.4cD | 63.6 ± 1.7E | |||
| Fraction 4 | 1717 ± 10 | 1462 | 1457 | ||
| 0 (control) | 100 ± 3.1A | 100 ± 3.9A | |||
| 200 | 100 ± 1.4A | 100 ± 4.9A | |||
| 400 | 100 ± 0.7A | 100 ± 3.8A | |||
| 600c | 89.9 ± 5.4B | 80 ± 2.7C | |||
| 800c | 86.4 ± 4.8BC | 77.2 ± 5.5D | |||
| 1000 | 83 ± 3.2C | 75.2 ± 2.5D | |||
| 1200c | 81.1 ± 4.2C | 72.3 ± 3.6D | |||
| 1400 | 80 ± 0.32C | 72 ± 5.7D | |||
| Fraction 5 | 1312 ± 60 | 488 | 1547 | ||
| 0 (control) | 100 ± 4.1A | 100 ± 3.1A | |||
| 200 | 100 ± 7.2A | 100 ± 2.6A | |||
| 400 | 100 ± 2.7A | 100 ± 2.7A | |||
| 600c | 62.9 ± 2.2B | 53.2 ± 4.5C | |||
| 800c | 33 ± 3.6D | 22.3 ± 3.9E | |||
| 1000c | 25 ± 1.3E | 17.3 ± 2.0F | |||
| 1200 | 14.4 ± 2.5FG | 10.4 ± 1.9G | |||
| 1400 | 12.2 ± 0.9G | 8.7 ± 0.7G | |||
| Doxorubicin | |||||
| 0.25 | 19.4 ± 4A | 15 ± 2.3B | |||
| 0.125 | 12.7 ± 2.4C | 8.3 ± 1.1D | |||
| 0.0625 | 9.9 ± 0.4D | 6.1 ± 0.9E |
aDifferent letters show significant differences among various concentrations of the crude extract and fractions 1 - 5.
bValues are expressed as mean ± SD.
cIndicates a significant difference between cell viability at 24 and 48 hours.
3.2. Inhibitory Effects
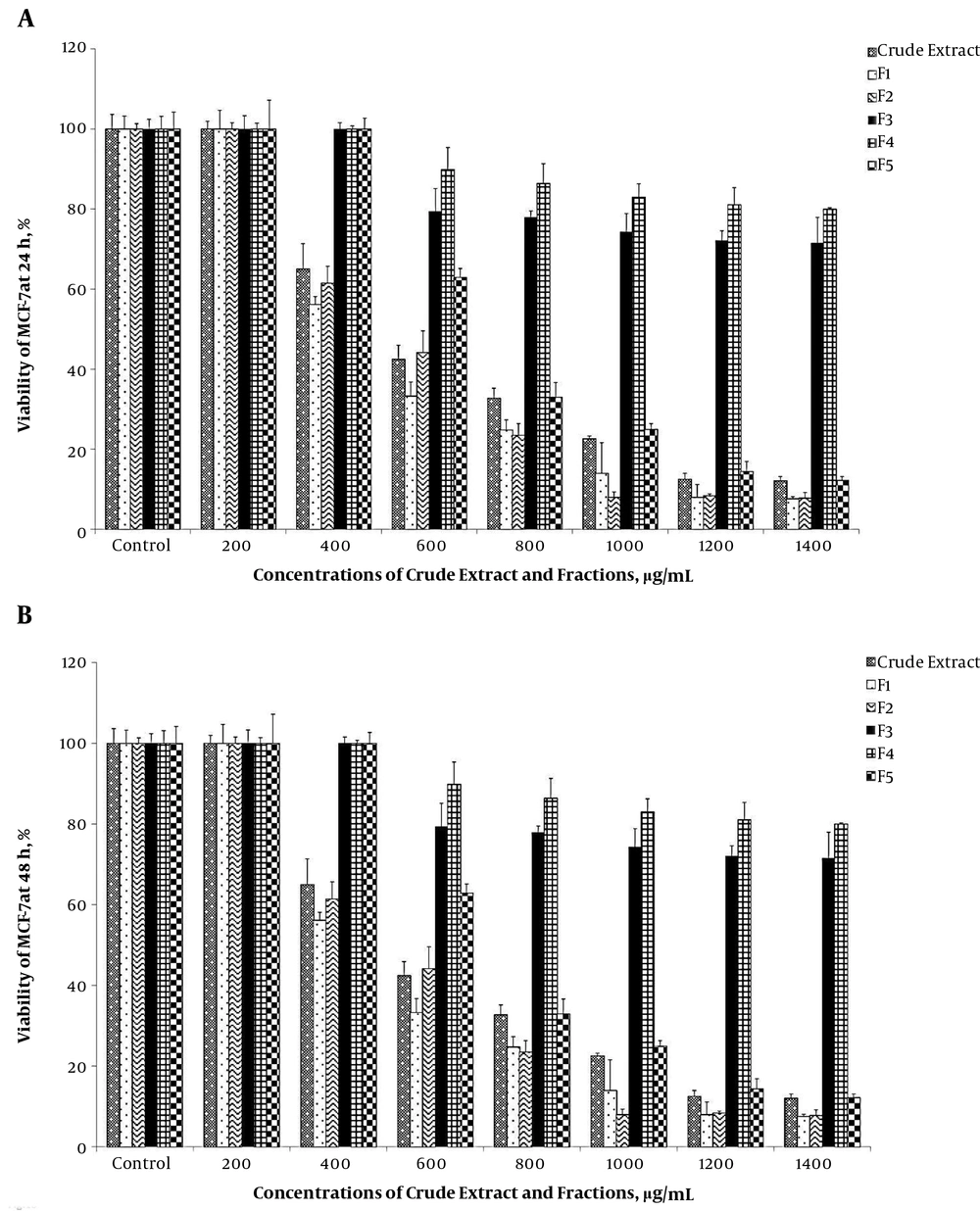
The results showed that the growth of breast cancer cell, MCF-7 was significantly inhibited by the crude extract and fractions 1 to 5 of the sea anemone oral disk (P < 0.05). Comparison of MCF-7 viability percentages between 24 and 48 hours against different concentrations of the crude extract and various fractions is presented in Table 1.
The MTT test showed that there was no significant difference between the control and the concentration of 200 μg/mL of the crude extract, or fractions 1 and 2 (Table 1). The MCF-7 viability decreased significantly from concentrations of 400 to 1400 μg/mL of the crude extract (P < 0.05) (Table 1). There was no significant difference between viability percentage of MCF-7 at three concentrations of 1000, 1200 and 1400 μg/mL (P > 0.05) (Table 1).
Fraction 1 significantly decreased the viability percentage of MCF-7 at the concentration of 400 μg/mL compared to the control (P < 0.05) (Table 1). No significant difference was recorded between the viability percentage of MCF-7 from 600 to 1400 μg/mL of fraction 1 (P > 0.05) (Table 1).
The result of MTT test for fraction 2 showed that viability percentage of MCF-7 was significantly decreased from 400 to 1000 μg/mL compared to the control (P < 0.05) (Table 1). The lowest cell viability percentage was at 1400 μg/mL of fraction 2 after 48 hours. There was no significant difference between MCF-7 viability percentage at three concentrations of 1000, 1200 and 1400 μg/mL (P > 0.05) (Table 1).
There was no significant difference between concentrations 200 and 400 μg/mL of fractions 3, 4 and 5 with the control in terms of viability of the MCF-7 cell line (P > 0.05) (Table 1).
A concentration of 600 μg/mL in fractions 3 and 4 significantly decreased viability of MCF-7 (P < 0.05) (Table 1); in addition, a significant difference was not observed between viability percentage of MCF-7 from 800 to 1400 μg/mL concentrations of fractions 3 and 4 (P > 0.05) (Table 1). In fraction 5, a significant decreasing trend was observed in the viability percentage of MCF-7 at 600 μg/mL and higher concentrations (P < 0.05) (Table 1).
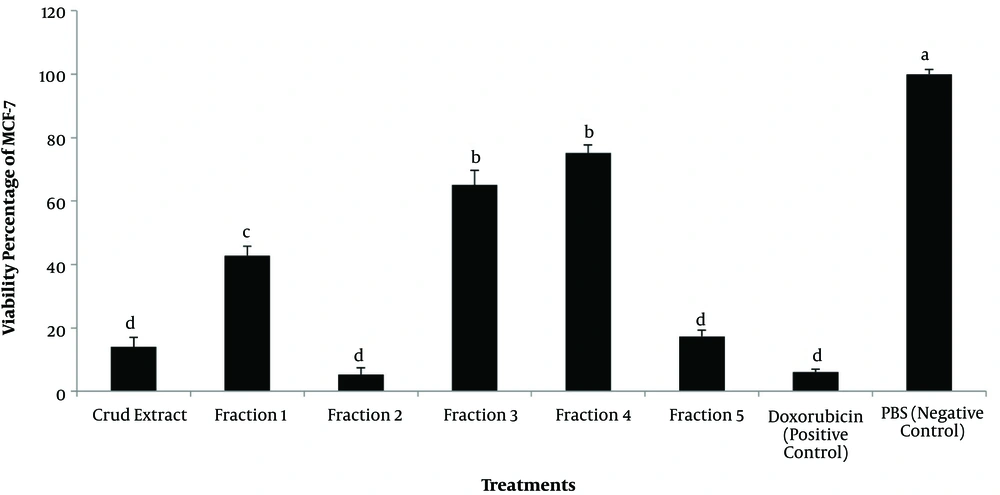
Comparison of the anticancer effects of the crude extract and fractions 1 - 5 in each individual concentration showed that the lowest viability percentage of MCF-7 was at 1400 μg/mL in fractions 1 to 5 after 24 and 48 hours (Figures 1a and 1b). Comparison of the most lethal concentration of anticancer drug (doxorubicin) and treatments on the growth of MCF-7 showed that fractions 2, 5, and crude extract as well as the anticancer drug, had similar effects (P > 0.05) (Figure 2).
Inhibitory effects of the highest doses of crude extract, fractions 1 up to 5 and controls from oral disk of sea anemone (according to the lethal concentration of each treatment) against the MCF-7. Different letters show significant differences among various concentrations of the crude extract and fractions 1 - 5.
3.3. Inhibitory Concentration Values (IC50)
The IC50 values for the crude extract and fractions 1 to 5 are presented in Table 1.
3.4. Reversed-Phase HPLC
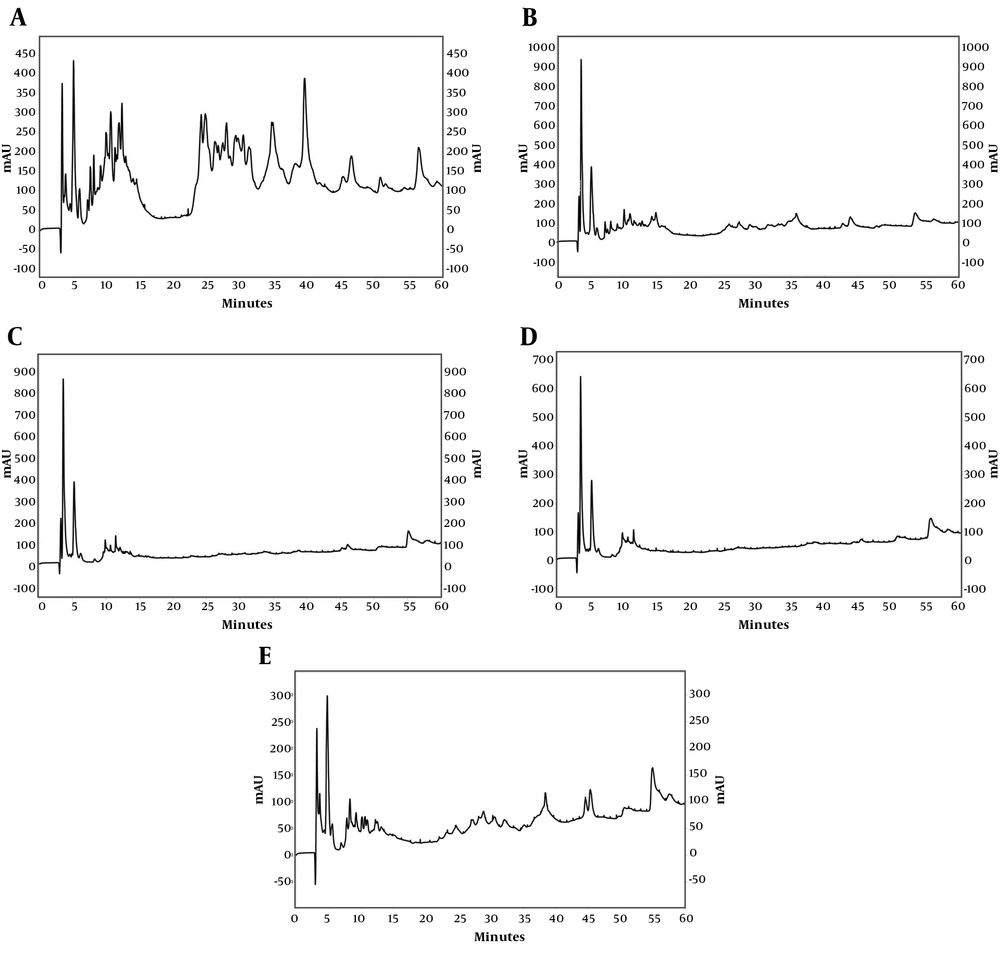
Reversed-phase HPLC analysis determined the protein/peptide fingerprints of S. haddoni, in terms of hydrophobicity. The RP-HPLC chromatogram of each fraction is illustrated in Figure 3. In all five fractions, a high absorbance intensity group of protein/peptide was separated before 16 minutes (~20% acetonitrile). Clearly, proteins/peptides were also separated, in fractions 1, 2 and 5, after 22 minutes (> 25% acetonitrile). On the other hand, fraction 1 and 5 exhibited a richer elution pattern compared to fraction 2, in this range. About 50% of fraction 2 and ~43% of fraction 5 were hydrophobic molecules that separated at higher acetonitrile percentages (Table 2).
| Retention Time (Fraction 1), Minutes | % ACN (Fraction 1) | Retention Time (Fraction 2), Minutes | % ACN (Fraction 2) | Retention Time (Fraction 3), Minutes | % ACN (Fraction 3) | Retention Time (Fraction 4), Minutes | % ACN (Fraction 4) | Retention Time (Fraction 5), Minutes | % ACN (Fraction 5) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.33 | 9.3 | 3.4 | 9.3 | 3.62 | 9.5 | 3.30 | 9.3 | 3.2 | 9.2 |
| 2 | 3.85 | 9.7 | 3.7 | 9.5 | 4.60 | 10.3 | 3.63 | 9.5 | 3.7 | 9.6 |
| 3 | 4.52 | 10.3 | 4.6 | 10.3 | 4.92 | 10.6 | 4.90 | 10.6 | 4.4 | 10.2 |
| 4 | 5.03 | 10.7 | 5.2 | 10.8 | 5.23 | 10.9 | 5.27 | 10.9 | 4.9 | 10.5 |
| 5 | 5.93 | 11.4 | 6.0 | 11.5 | 6.22 | 11.7 | 6.27 | 11.7 | 5.6 | 11.2 |
| 6 | 7.12 | 12.4 | 7.2 | 12.5 | 9.60 | 14.5 | 9.82 | 14.7 | 7.9 | 13.0 |
| 7 | 7.52 | 12.8 | 7.6 | 12.8 | 9.88 | 14.7 | 10.43 | 15.2 | 8.3 | 13.4 |
| 8 | 8.05 | 13.2 | 8.1 | 13.2 | 10.67 | 15.4 | 10.62 | 15.3 | 9.0 | 14.0 |
| 9 | 8.65 | 13.7 | 8.4 | 13.5 | 11.43 | 16.0 | 11.20 | 15.8 | 9.3 | 14.2 |
| 10 | 9.03 | 14.0 | 9.1 | 14.0 | 12.10 | 16.6 | 11.47 | 16.0 | 10.2 | 15.0 |
| 11 | 9.85 | 14.7 | 9.5 | 14.4 | 12.55 | 16.9 | 12.35 | 16.8 | 10.7 | 15.4 |
| 12 | 10.20 | 15.0 | 10.1 | 14.9 | 12.75 | 17.1 | 13.12 | 17.4 | 11.0 | 15.6 |
| 13 | 10.55 | 15.3 | 10.6 | 15.3 | 12.95 | 17.3 | 13.63 | 17.8 | 11.7 | 16.2 |
| 14 | 11.20 | 15.8 | 10.9 | 15.6 | 13.18 | 17.5 | 14.80 | 18.8 | 12.0 | 16.4 |
| 15 | 11.42 | 16.0 | 11.6 | 16.1 | 13.62 | 17.8 | 55.37 | 52.5 | 12.3 | 16.7 |
| 16 | 11.80 | 16.3 | 12.4 | 16.8 | 14.75 | 18.8 | 56.27 | 53.2 | 12.5 | 16.9 |
| 17 | 12.22 | 16.7 | 12.8 | 17.1 | 14.90 | 18.9 | 57.85 | 54.5 | 13.2 | 17.4 |
| 18 | 12.78 | 17.1 | 13.1 | 17.3 | 15.20 | 19.1 | 13.6 | 17.8 | ||
| 19 | 13.88 | 18.0 | 13.2 | 17.5 | 46.07 | 44.7 | 14.5 | 18.6 | ||
| 20 | 14.40 | 18.5 | 14.2 | 18.3 | 55.15 | 52.3 | 14.9 | 18.9 | ||
| 21 | 15.48 | 19.4 | 14.8 | 18.8 | 55.88 | 52.9 | 24.6 | 26.9 | ||
| 22 | 15.60 | 19.5 | 15.6 | 19.5 | 57.867 | 54.5 | 27.0 | 28.9 | ||
| 23 | 22.07 | 24.8 | 16.2 | 19.9 | 58.267 | 54.9 | 28.2 | 29.9 | ||
| 24 | 24.02 | 26.4 | 25.7 | 27.9 | 28.9 | 30.5 | ||||
| 25 | 24.63 | 27.0 | 26.2 | 28.2 | 30.3 | 31.7 | ||||
| 26 | 26.05 | 28.1 | 26.5 | 28.5 | 30.6 | 31.9 | ||||
| 27 | 26.55 | 28.6 | 27.2 | 29.1 | 32.1 | 33.2 | ||||
| 28 | 27.23 | 29.1 | 28.2 | 29.9 | 38.4 | 38.4 | ||||
| 29 | 27.78 | 29.6 | 28.8 | 30.4 | 39.7 | 39.5 | ||||
| 30 | 28.28 | 30.0 | 29.2 | 30.8 | 44.6 | 43.5 | ||||
| 31 | 29.15 | 30.7 | 29.6 | 31.1 | 45.3 | 44.1 | ||||
| 32 | 29.48 | 31.0 | 30.2 | 31.6 | 55.0 | 52.1 | ||||
| 33 | 30.27 | 31.6 | 30.8 | 32.1 | 56.0 | 53.0 | ||||
| 34 | 31.12 | 32.3 | 31.5 | 32.7 | 57.5 | 54.3 | ||||
| 35 | 34.50 | 35.1 | 32.1 | 33.2 | 57.9 | 54.5 | ||||
| 36 | 34.68 | 35.3 | 33.1 | 34.0 | ||||||
| 37 | 35.98 | 36.4 | 33.6 | 34.4 | ||||||
| 38 | 38.00 | 38.1 | 34.7 | 35.3 | ||||||
| 39 | 38.62 | 38.6 | 34.9 | 35.4 | ||||||
| 40 | 39.38 | 39.2 | 35.7 | 36.2 | ||||||
| 41 | 41.62 | 41.1 | 37.2 | 37.4 | ||||||
| 42 | 42.38 | 41.7 | 37.3 | 37.5 | ||||||
| 43 | 43.65 | 42.7 | 42.7 | 41.9 | ||||||
| 44 | 45.12 | 44.0 | 43.8 | 42.9 | ||||||
| 45 | 46.35 | 45.0 | 53.5 | 50.9 | ||||||
| 46 | 48.65 | 46.9 | 54.6 | 51.8 | ||||||
| 47 | 48.83 | 47.0 | 54.9 | 52.1 | ||||||
| 48 | 50.65 | 48.6 | 55.4 | 52.5 | ||||||
| 49 | 51.42 | 49.2 | 56.2 | 53.1 | ||||||
| 50 | 56.32 | 53.3 | 56.4 | 53.3 |
4. Discussion
In the present study, inhibitory effects of the crude extract obtained from S. haddoni oral disk and its fractions on growth of MCF-7 was further enhanced with increasing dose and time of treatments.
The sea anemone Heteractis magnifica toxin has induced apoptosis in the lung and breast cancer cells (18, 19). It was found that H. magnifica toxin induced apoptosis in breast cancer cells T47D and MCF-7 (19). The lung cancer cell A549 treated with of H. magnifica toxin has shown a delayed progression through the cell cycle and accumulation of cells in G0/G1 phase (18). Soletti et al. (13) demonstrated that two cytolysins extracted from the sea anemone, BC2 and EqTX-II, induced cytotoxic effects against the human glioblastoma cell lines U87 and A127. In the present study, oral disk of S. haddoni was separated for the extraction of toxins due to the high density of nematocyst on the oral disk. Since the sea anemone toxins that possess cytotoxic effects have a proteinaceous nature (20, 21), accordingly, an aqueous solvent, PBS, was used to extract the proteins from the oral disk of the sea anemone.
Based on the molecular weight of cytolysin peptides, sea anemone toxins can be divided into four groups (7): 5 - 8 kDa peptides with antihistamine activity; 20 kDa pore-forming proteins; 30 - 40 kDa cytolysins; and 80 kDa cytolysins. In the present study, fraction 2 (30 - 100 kDa) and fraction 5 (< 3 kDa) of S. haddoni showed the highest lethality percentage of MCF-7 than the other fractions. The reason for the high rate of cell lethality in fractions 2 and 5 can be attributed to the high levels of proteins or peptides. Reversed-phase HPLC results also showed that fractions 2 and 5 had the highest profiles. In comparison with fraction 2, fraction 5 could be a better candidate for extraction of the anticancer drug, due to the lower molecular weight and possibility of easier purification of its components for pharmacological studies.
A reduction in the viability of human glioblastoma cells U87 treated with the sea anemone (Bunodosoma caissarum) toxin was observed at a concentration of 1000 μg/mL (13). In another study, the effective lethal concentration of H. crispa and H. magnifica toxins on the cancer cells, A549, T47D and A431, was 40 μg/mL (14). In the current study, concentrations of 1200 and 1400 μg/mL of different fractions of S. haddoni had the most inhibitory effect on the growth of MCF-7. It may also be noted that low concentrations of the toxins or drugs is an advantage due to fewer side effects. Therefore, the concentration of 1200, in comparison with 1400 μg/mL, is more desirable for use in the purification processes of an effective anticancer agent from the sea anemone.
In conclusion, the extracts and different fractions obtained from the oral disk of the sea anemone had an inhibitory effect on the growth of the breast cancer cell MCF-7. Fraction 5 with a concentration of 1200 to 1400 μg/mL than the other fractions showed the best anticancer effect. Therefore, fraction 5 is proposed as an appropriate candidate for purification of its peptides for pharmacological studies.