1. Background
A cut or hole in the normal anatomical structure of epithelial tissue is defined as a wound. It can occur at different degrees of severity (1). The complete restoration of internal and external structures of the injured area is wound healing. During this process, there are many interactions between different types of cells, structural proteins, growth factors, and other molecules. Furthermore, mast cells and macrophages play an important role in the healing process of wounds (2). The classic model of wound healing is divided into three, some belief four, sequential, yet overlapping phases: hemostasis (not considered a phase by some authors), inflammation, proliferation, and remodeling (3). These four stages are considered to take place on days 3, 7, 14, and 21 after the injury, which are critical days in any wound healing processes.
In Iranian traditional medicine, AnbarNesa smoke, in Chinese medicine, the tea leaf extract, and in ancient Egypt, honey and gum of trees were used for wound healing purposes. Nowadays, various kinds of ointments and drugs are being used to heal open wounds, each with advantages, limitations, and side effects (4-6). Since traditional and natural remedies have fewer side effects than similar synthetic products, many prefer to use the herbal drugs instead of chemical ones (7-10). Thus, the application of traditional medicine persuades researchers to produce a range of promising new drugs with higher activity and/or lower toxicity (11). The process of wound healing is promoted by several natural products (12) and herbal extracts, which are composed of active components such as triterpenes, alkaloids, and flavonoids (13) and even biomolecules (14). These agents typically influence one or more phases of the healing process (15).
AnbarNesa (or AnbarNasara) is the female donkey dung mostly collected after the labor and in early springtime. Famous Iranian traditional physician, Abu Ali Sina or Ibn Sina (980 - 1037 CE), known as Avicenna, used AnbarNesa smoke to treat some infections of the female reproductive tract (i.e., vaginitis, uteritis, etc.), and also reduce premenstrual syndrome and hemorrhage (16). He also treated the puerperal infection and menometrorrhagia with AnbarNesa; thus, its name comes from Anbar (smoke) and Nesa (women). In Iranian traditional medicine, AnbarNesa smoke was also widely used to treat skin ulcers, chickenpox blisters, oral inflammatory lesions such as aphthous ulcers or inflammations such as otitis media and externa (17, 18).
2. Objectives
Nowadays, it is worthy of extensive research about AnbarNesa effects and its other possible medical applications, along with the mechanisms of action in the abovementioned complications. Therefore, the current study aimed at investigating the effect of AnbarNesa on wound healing in an animal-based model for the first time, in order to develop an effective wound healing drug for modern medicine using natural products.
3. Methods
3.1. Drugs
AnbarNesa was primarily burned in solid form and the smoke was analyzed by gas chromatography-mass spectrometry (GC-mass) and more than eight substances were identified with anti-inflammatory effects such as dichloromethane, acetic acid, limonene, etc., some antibacterial agents; i.e., phenol, licochalcone A, carvacrol, cresol, etc., some anti-neoplasms such as prospidium chloride (19), and some others (monoterpens, diterpens, sesquiterpenes, antioxidants, etc.). These ingredients assured authors that AnbarNesa may have promising effects on wound healing. Accordingly, to make a new drug, AnbarNesa smoke was dissolved in propylene glycol (Merck; Germany) considering the weight/volume method (it was neither soluble in water nor in alcohol). Solving the smoke in propylene glycol for the first time resulted in an invented solution product named ANNAS. After serial dilutions, first, the antibacterial activity of ANNAS was examined and it was found that it has a significant activity against all bacterial species (except Enterococcus faecalis) even in very low concentrations (20). Then the non-toxic doses of ANNAS were measured for fibroblast cells by methyl thiazolyl tetrazolium (MTT) method (21) and its non-toxic dose was used for the intervention groups in the current study, while its solvent, propylene glycol, was employed for the control groups.
3.2. Animals
All study protocols were approved by the deputy for animal research and the Ethics Committee of Shahid Beheshti University and were conducted in accordance with the internationally accepted principles for laboratory animal use and care (International Association for the Study of Pain, 1983) documented in Iran national community guidelines and the Institutional Animal Welfare Law.
Consequently, 16 adult male Wistar rats, weighing 180 - 250 g, were housed in animal cages at room temperature of 22 ± 0.5°C with a 12 - 12 hour light/dark cycle and free access to water and food. After acclimation, four rats were randomly selected for the pilot study on days 3, 7, 14, and 21, which are critical days in the wound healing process. And 12 rats were randomly divided into two groups (n = 6) for the main study on days 14 and 21.
3.3. Wound Creation
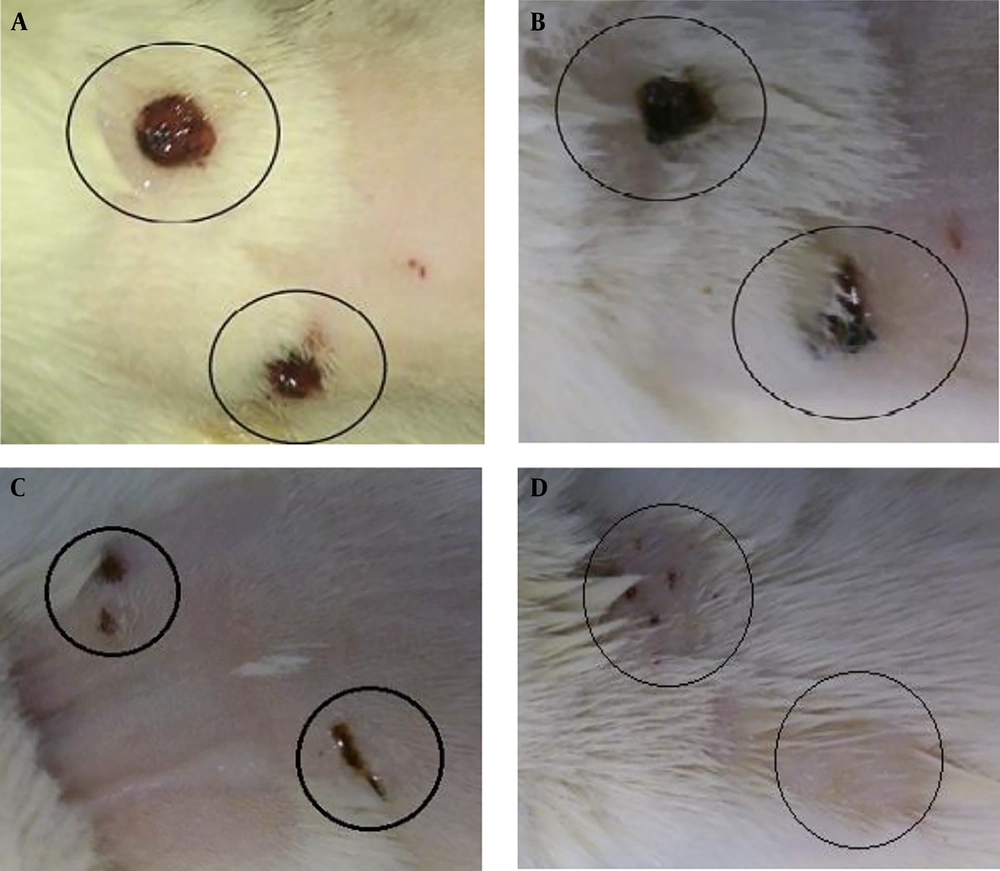
After brief anesthesia with ketamine (40 mg/kg) and xylazine HCL (4 mg/kg), the dorsal skin of each rat was shaved and sterilized with 70% ethanol in a small area. Subsequently, two 5-mm full-thickness lesions with 15 mm distance were created on either side of the vertebral column by a biopsy punch device (Figure 1).
3.4. Wound Treatment
The lesions were randomly selected for the intervention or control group. The lesions in the intervention groups were treated with one drop of AnbarNesa solution (ANNAS), and the control ones were given the same amount of its solvent, propylene glycol, once a day every morning. At the end of each period of the experiment, the rats were sacrificed after CO2 inhalation, and wounds were excised entirely on their longest diameter for histological study.
3.5. Histological Evaluation
The dissected samples were fixed by 10% formalin for at least 24 hours. Then paraffin blocks were prepared and sectioned. The obtained sections were stained using hematoxylin and eosin (H & E) and smooth muscle actin (SMA), immunohistochemistry (IHC) method, and then examined under a light microscope (Olympus E400, Japan).
In H & E staining, the histological examination included the type of inflammation (acute or chronic), the intensity of inflammation (scores 0 < 10, 1 < 10 - 30, 2 < 30 - 50, 3 > 50), re-epithelialization (+/-), type of connective tissue (granulation tissue, fibrosis, and necrosis), and the continuity or the interruption of the epithelium.
In SMA (IHC method), the sections were stained with anti-SMA antigen for myofibroblast detection and the number of myofibroblasts was counted in each field of an optical microscope with 400× magnification. Three dermis layers were randomly selected from surface to depth and the average number of counted myofibroblasts in each field of view was calculated.
3.6. Pilot Study
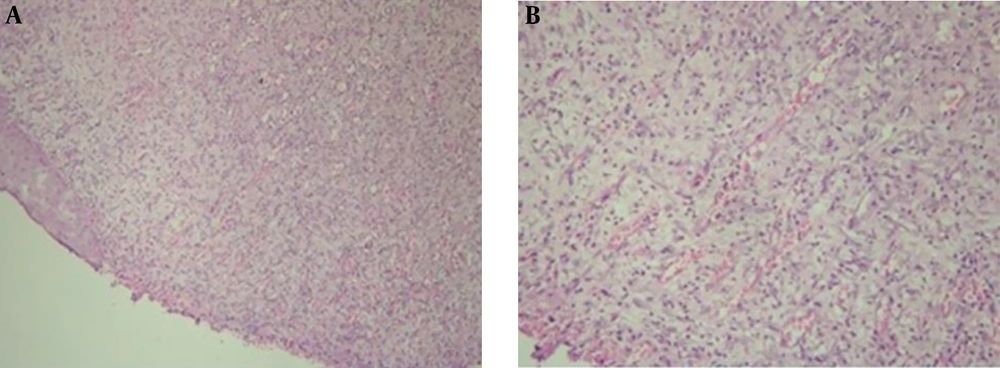
In this elementary part of the study, the manner of connective tissue, also the type and degree of inflammation were approximately similar in H & E staining of the ANNAS-treated sections compared with those of the control ones on all experimental days (3, 7, 14, and 21) (Figure 2).
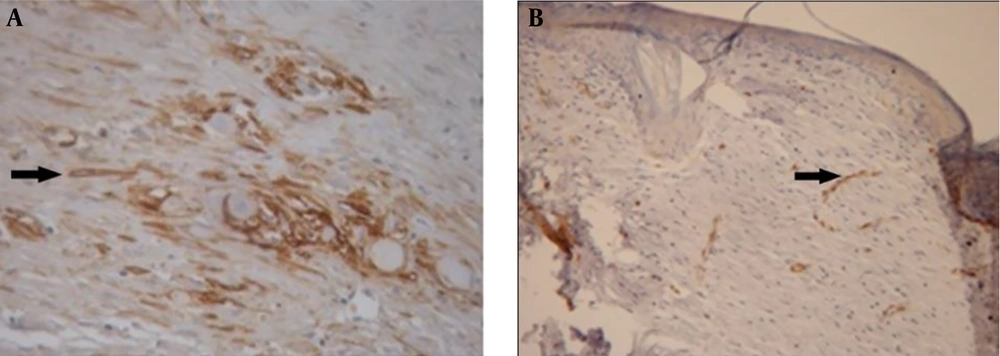
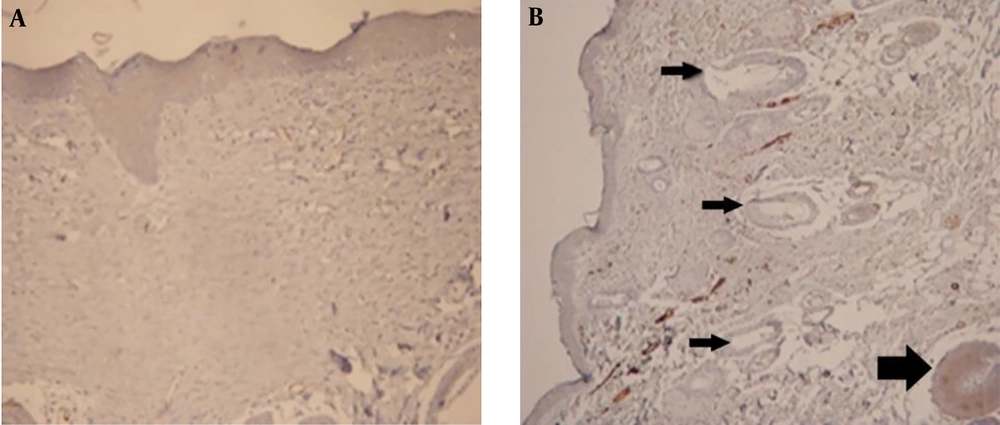
Thus, H & E staining method was omitted in the main study, but SMA was considered as the proper technique for this experiment. However, with IHC method, the number of myofibroblasts was considerably different on days 14 (Figure 3) and 21, but there were no significant differences between days 3 and 7; therefore, these days were also excluded from the main study.
3.7. Main Study
Twelve male Wistar rats were equally divided into two groups to be studied on days 14 and 21. After treatment, the histological blocks were prepared and with SMA (IHC method), the mean number of myofibroblasts in three fields of the surface, as well as three fields of deep dermis, was measured and reported as the number of myofibroblasts in each zone of the lesions.
3.8. Statistical Analysis
The means ± SEM were used to compare the control and ANNAS-treated lesions in surface and deep dermis (individually and totally) on days 14 and 21. Paired samples t-test was performed by SPSS version 16 (IBM, United States). The significance levels were reported as: *, P ≤ 0.05;**, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
4. Results
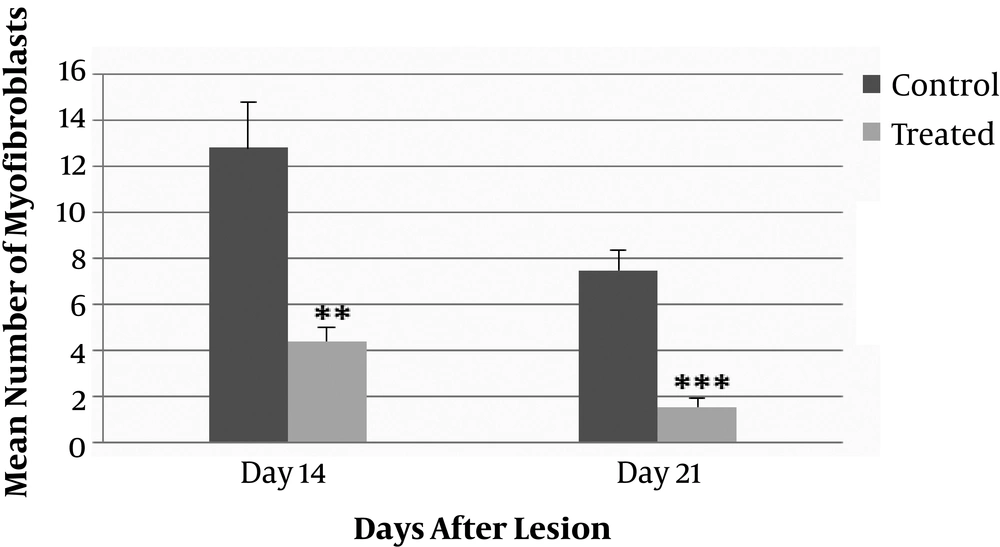
Inflammatory cells were generally similar in both the control (propylene glycol) and ANNAS-treated wounds. But, the numbers of myofibroblasts were significantly lower in surface and deep layers of dermis on days 14 and 21 in the ANNAS-treated lesions compared with those of the controls. Moreover, there were no significant differences between the surface and deep layers of dermis with regard to the number of myofibroblasts.
4.1. The Wound Surface Layer of Dermis
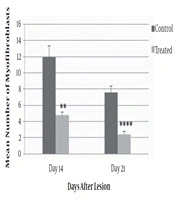
As shown in Figure 4, the mean number of myofibroblast cells in the wound surface layers of dermis in the ANNAS-treated groups compared with those of the control groups indicated a significant difference on days 14 (P value = 0.03) and 21 (P value = 0.003).
4.2. The Wound Deep Layers of Dermis
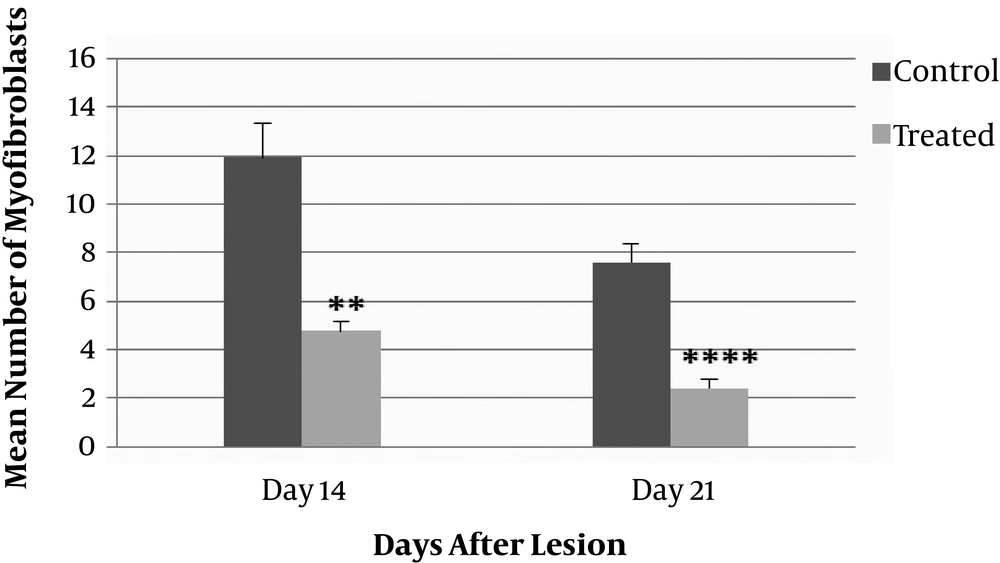
The number of myofibroblasts in the wound deep layers of dermis in the ANNAS-treated groups and their controls are shown in Figure 5. The comparison between the groups illustrated a significant difference on day 14 (P value = 0.004) and more significant differences on day 21 (P value = 0.001).
4.3. The Surfaces and Deep Layers of Dermis
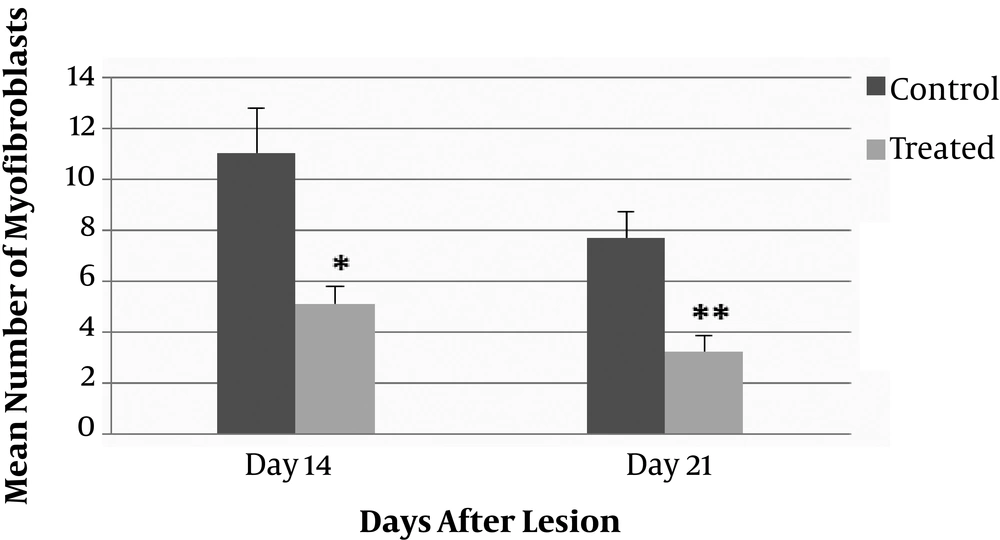
According to the results, there was a significant difference in the number of myofibroblast cells in the entire dermis between the ANNAS-treated lesions and the controls on days 14 (P value = 0.002) and 21 (P value = 0.0001). The results are shown in Figure 6.
The number of myofibroblasts in the ANNAS-treated lesions were approximately three folds lower than those of the control ones on days 14 and 21 (Figures 3 and 7).
Moreover, on day 21 in all of the ANNAS-treated samples, hair follicles and sebaceous glands were observed (Figure 7B) that can be a proof for accelerated wound healing, even grossly (Figure 8) by AnbarNesa solution, the smoke of which was traditionally used for the same purpose.
5. Discussion
Many investigations worldwide on traditional medicine and herbal drugs show acceptable results of using such drugs in medical treatments. The various kinds of plants and the customary treatment regimens can improve the healing process without causing any serious side effects. However, there are some differences among the results.
Khalil et al. (22), showed that Jordan plants had positive effects on the treatment of lesions in mice. Furthermore, Asif et al. (23), showed a significant promotion of wound healing activity by means of both the aqueous and methanolic root extracts of Berberis lyceum (a Pakistani plant). Gal et al. (24), showed that an aqueous extract of Atropa belladonna positively modulated early phases of skin wound healing with no significant antimicrobial properties. They believed that the effect of the plant can probably be attributed to the acceleration of angiogenesis and its anti-inflammatory properties. In another study, Nayak et al. (25), revealed that Morinda citrifolia enhanced wound contraction and decreased epithelialization time. Olugbuyiro et al. (7), showed that chloroform extract of Flabella riapaniculata had a potential anti-infective and wound healing effect. Wu et al. (26), also showed that the extract from the Chinese medicinal herb, Polygonum cuspidatum, possessed wound healing activity, and thus provided the evidence for its traditional use. It is proved by many other studies that plants and natural sources can improve the treatment of lesions. However, their mechanism of action is unclear thus far and needs further investigations.
AnbarNesa is collected in springtime and it seems that there could be a relationship between spring plants and the medical efficacy of this remedy. Some substances identified on GC-mass analyses of AnbarNesa were: marjoram, licochalcone A (licorice root), octadecane (a medicinal plant applied for the treatment of infectious diseases), and limonene (obtained from the fresh shoots of the flowering plant, Mentha piperita) (19). Thus, it was assumed that all these herbal products may play a role in the efficacy of AnbarNesa. On the other hand, in Iranian traditional medicine, AnbarNesa is used for wound healing purposes as an antibacterial agent, although it is collected from the nature and may have contaminations. Therefore, ANNAS was first examined in culture media and surprisingly, it was free from any microbial contaminations and even had antibacterial activities, almost similar to that of chlorhexidine (20). Thus, the authors were assured that ANNAS may have promising effects on wound healing.
Wound healing is an intricate process in which the skin (or another organ/tissue) repairs itself after injury (15). In normal skin, the epidermis and dermis exist in steady-state equilibrium form a protective barrier to the external environment. Once the protective barrier is broken, the physiologic process of wound healing is immediately set in motion. Wound healing has four stages: (1) re-epithelialization of wound surface; (2) migration of fibroblasts for collagen configuration; (3) formation of granulation tissue; and (4) scar contraction. These four stages are correlated or sometimes overlapped with each other (27). Upon injury to the skin, a set of complex biochemical events takes place in a closely orchestrated cascade to repair the damage (28).
A fibroblast is a type of cell that synthesizes the extracellular matrix, the structural framework (stroma) for animal tissue, and plays a critical role in wound healing. Studies show that fibroblasts are involved in formation of collagen and the size of extracellular matrix. Additionally, they participate in the repair process by differentiating into myofibroblasts, which are cells involved in the inflammatory response to injury. Myofibroblasts migrate to the sites of injury where they produce cytokines, thus enhancing the inflammatory response (29). In this study, no inflammation difference may be due to decrease in myofibroblast formation (Figure 3B).
A myofibroblast is a cell in between a fibroblast and a smooth muscle cell in phenotype. Myofibroblasts can contract by the use of smooth muscle type, actin-myosin complex, rich in a form of actin called alpha-smooth muscle actin. These cells are then capable of accelerating wound repair by contracting the edges of the wound (28). Therefore, myofibroblast is a specialized contractile cell type responsible for wound closure, tissue contraction, and scarring. When healing is complete, these cells are lost through apoptosis and it is suggested that in several fibrotic diseases (i.e., liver cirrhosis, kidney fibrosis, and retroperitoneal fibrosis), this mechanism fails to work, leading to persistence of the myofibroblasts, and consequently expansion of the extracellular matrix (fibrosis) and contraction (30). Similarly, in wounds that fail to resolve and become keloid or hypertrophic scars, myofibroblasts may persist, rather than disappearing by apoptosis (31).
In the present study, the positive effect of AnbarNesa was observed during lesion recovery (Figures 3B, 7B, and 8B-D). This significant effect could be related to the probable ability of AnbarNesa to influence angiogenesis and any other helping factor, i.e. vascular endothelial growth factor (VEGF), for blood vessels formation that plays an important and essential role in feeding lesion and accelerating its improvement; it may also activate the apoptosis or be involved in other processes of wound healing. It probably affects growth factors, interleukins, and cytokines. At first, it was hypothesized that there is a connection between AnbarNesa and transforming growth factor beta (TGF-β) that induces the migration of fibroblasts and also collagenesis and thus acceleration of healing. In addition, TGF-β can avert substances that can cause harm to the external matrix layer of the cells. As a point, the increase in the number of myofibroblasts, mast cells, and macrophages results in scarring that indicates lesion improvement (32-34). But, contradictory to the above mentioned facts, lower number of myofibroblasts and limited scars were observed.
The exact mechanism of scarring is unknown. Scars may be caused during nutrients delivery similar to inflammatory cells migration to the lesion area by means of blood vessels that removes external matrix from that place. Therefore, in the current study, there may be another mechanism for angiogenesis (VEGF) or some other factors may be involved in the myofibroblast decrescendo in the ANNAS-treated group such as acceleration of apoptosis. More investigations are needed to understand the real mechanisms involved in this process.
In the present study performed for the first time on the effects of AnbarNesa on relieving the skin lesions, a decrease was observed in the number of myofibroblasts on days 14 (Figure 3B) and 21, which means that AnbarNesa was able to prevent the keloid formation and, consequently, the scar creation. Also, dermal appendages were observable on day 21 (Figure 7B) that was a good sign of recovery. Thus, AnbarNesa could accelerate wound healing by speeding up the hair follicle formation and sebaceous glands generation. Could it be via enhancing the apoptosis? Does it have any effect on growth factors and angiogenesis? What mechanisms are involved in this process? Future studies may answer some of these questions and authors hope to produce a new useful drug to treat skin lesions.
5.1. Conclusions
It can be concluded that the smoke of AnbarNesa or its solution, ANNAS, has a significant ability to accelerate lesion recovery process with minimum scar formation. Since it was the first study on AnbarNesa solution, further studies should be conducted to understand the exact mechanisms of the effects of ANNAS on wound healing processes.