1. Background
Diabetes mellitus is a group of metabolic disorders characterized by increased blood glucose levels (hyperglycemia) caused by defect in insulin secretion, cell response to insulin, or both. This leads to wide changes in the cellular metabolism of different tissue such as liver, muscle, kidney, brain, and fat cells (1, 2). Due to changes in people’s lifestyle and dietary habits, in recent years the prevalence of diabetes significantly increased (3). If diabetes left untreated, it can cause damage to different body organs. The goal of diabetes treatment is to control blood sugar and associated complications such as hypertension and hyperlipidemia, which play an important role in reducing long-term complications of diabetes.
It is a long time that medicinal plants are commonly used to treat or control diabetes (4). In traditional medicine, gum resin derived from the root and rhizome of Ferula asafoetida is used to treat diabetes (5). In different countries, particularly India, Afghanistan, and Iran, asafoetida is traditionally used to treat complications such as asthma, epilepsy, abdominal pain, bloating, etc. (6, 7). It is observed that there are many different active compounds such as ferulic acid, farnesiferol A and C, saradaferin, and foetidin in F. asafoetida gum (7-9). Furthermore, since the gum contains different antioxidant compounds, it could reduce the level of intracellular free radicals and consequently strengthen and protect the remaining beta cells and increase insulin secretion, and consequently improve pancreatic function and complications of diabetes (10).
In recent years, the development of animal models allows the researchers to have a closer look at antidiabetic mechanism of medicinal herbs. Injecting a single dose of streptozotocin (STZ) can induce type 1 diabetes in rats (11). Abu-Zaiton demonstrated that an aqueous extract of F. asafoetida significantly reduced blood glucose and increased serum insulin levels by preserving pancreatic beta cells in alloxan-induced diabetic rats (12). Moreover, the effect of asafoetida on stimulating the pancreatic beta cells and their regeneration is reported (5).
Due to the common use of medicinal plants as complementary and alternative medicine in the world (13-18), more studies are required to support the therapeutic effects of these plants. According to the best of authors` knowledge, the antidiabetic effect of ethyl acetate extract of F. asafoetida is not yet reported.
2. Objectives
Therefore, the current study aimed at investigating the effect of ethyl acetate extract of F. asafoetida gum on the serum glucose level and lipid profile in streptozotocin-induced diabetic rats.
3. Methods
3.1. Laboratory Animals
The current experimental study was conducted on 40 male Wistar rats (200 - 250 g) purchased from Mashhad Razi Institute (Mashhad, Iran). All animals were housed under similar conditions (22 ± 2°C, 45% humidity with a 12:12-hour light/dark cycle) and easy access to standard pellet diet and tap water. All the tests were performed according to the Guide for the Care and Use of Laboratory Animals (NIH America, Publication No. 80-23, revised 1996) and all methods and protocols were approved by the Research Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran (ethic code: Medsab.Rec.93.77).
3.2. Extract Preparation
Ferula asafoetida gum resin was purchased from a local medicinal plants market (Sabzevar, Iran) and its authenticity was confirmed by Dr. Rezaee-Seresht H. the medicinal plant expert at Incubation Center of Sabzevar University of Medical Sciences. To prepare the ethyl acetate extract, initially 200 g of asafoetida was crushed and powdered. Then extraction was conducted by percolation, using ethyl acetate as a solvent. The extract was kept in the dark until evaporation of the solvent. At the end, 12 g of concentrated oily extract was achieved and kept in 10°C. Before injection, the extract was dissolved in 5% Tween 80 in distilled water.
3.3. Induction of Diabetes in Rats
Diabetes was inducted by intraperitoneal injection of a freshly prepared dose of streptozotocin (60 mg/kg in 0.1 M sodium citrate buffer, pH 4.5) to the fasting rats (the day 0). Three days later (the day 3), blood samples were taken from the retro-orbital sinus and serum blood glucose level was measured using a glucometer. Animals with blood glucose over 250 mg/dL were considered as diabetic.
3.4. Grouping and Treatment
Animals were randomly divided into five groups (eight rats per group) as follows: group I, healthy; group II, diabetic control; groups III - V, diabetic + asafetida, 25, 50, and 100 mg/kg, respectively (19). The asafoetida extract was intraperitoneally injected once daily for a 28-day period (from the days 3 - 31). The diabetic control group received 5% aqueous Tween 80 as vehicle by the same manner. In addition, the weight changes of animals were monitored during the experiment by a digital scale.
3.5. Blood Sampling
Two milliliters of blood were collected from tail vein of each animal after 12 hours fasting; before induction of diabetes (the day 0) and then, on the days 17 and 31 of the experiment. Blood samples were centrifuged at 1200 g for 10 minutes using benchtop centrifuge. The prepared serum samples were kept at -80°C until analysis. Serum glucose, triglycerides, cholesterol, and high-density lipoprotein (HDL) levels were measured using Roche Hitachi 911 automatic analyzer and commercially available kit. Low-density lipoprotein cholesterol (LDL-C) concentration was calculated using the Friedewald equation (20).
3.6. Statistical Analysis
Data analysis was performed with SPSS version 19 (Chicago, USA). The normality of the data was evaluated using the Shapiro-Wilk test. The results were expressed as mean ± standard error (SE). The intragroup changes over time were assessed by mixed model ANOVA. In case of significant differences (P < 0.05), the results were further compared by appropriate simple effects analyses and Bonferroni post-test.
4. Results
4.1. Effect of Ethyl Acetate Extract of Asafoetida on Serum Glucose Level
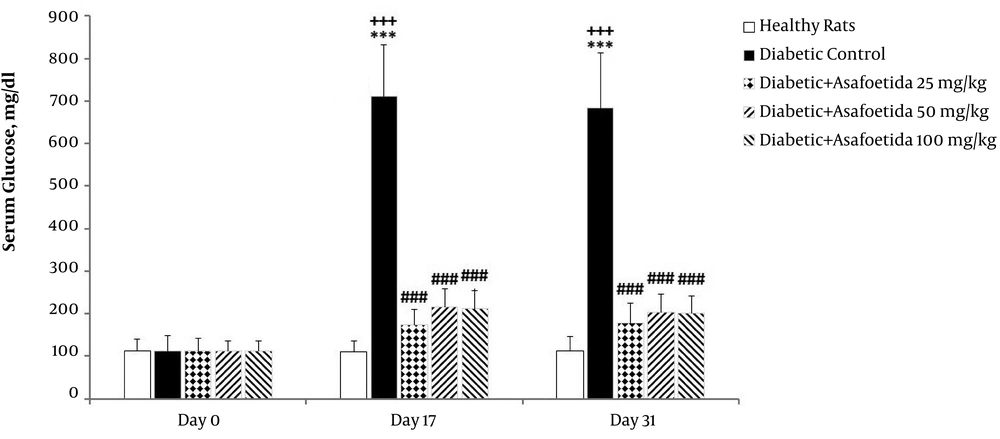
The 3 (time: days 0, 17, and 31) × 5 (groups: healthy rats, diabetic control, diabetic + asafoetida 25, 50, 100 mg/kg) mixed model ANOVA revealed significant main effects of time [F (2, 70) = 248.33, P < 0.001], treatment [F (4, 35) = 148.54, P < 0.001], as well as significant time × treatment interaction [F (8, 70) = 99.31, P < 0.001] on the glucose levels (Figure 1).
Effect of asafetida extract on the glucose level (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from the day 3 to 31 after administration of a single dose of streptozotocin. Serum glucose level was measured on the days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. ***P < 0.001 vs. healthy group on each day; ###P < 0.001 vs. diabetic control group on each day; +++P < 0.001 vs. the day 0 in each group.
In comparison with healthy rats, simple effects univariate analysis of groups for each day revealed a significant increase of glucose level for diabetic controls on the days 17 and 31 (P < 0.001). Meanwhile, the glucose level of the groups receiving extract was significantly lower than that of diabetic controls on the days 17 and 31 (P < 0.001). There was no significant difference between groups on the day 0.
Multivariate simple effect analysis of days for each group revealed no significant change in glucose level for healthy rats and groups receiving asafoetida (P > 0.05), but a significant change was observed for the diabetic group. Multiple comparisons with corrected Bonferroni test for the diabetic group indicated that serum glucose level significantly increased on the days 17 and 31 in comparison to the day 0 (P < 0.001). Taken together, these results indicated that serum glucose level markedly increased in animals following STZ injection, and asafoetida treatment could decrease its level.
4.2. Effect of Ethyl Acetate Extract of Asafoetida on Cholesterol
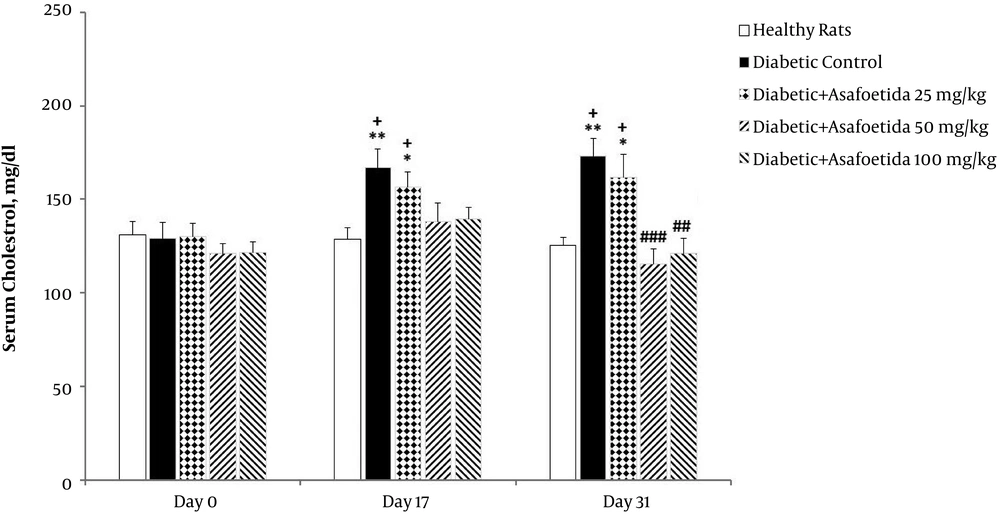
The 3 (time) × 5 (groups) mixed model ANOVA revealed significant main effects of time [F (2, 70) = 6.13, P = 0.03], treatment [F (4, 35) = 11.16, P < 0.001], as well as significant time × treatment interaction [F (8, 70) = 0.77, P > 0.05] on the cholesterol levels (Figure 2).
Effect of asafetida extract on the serum cholesterol level (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from the day 3 to 31 after administration of a single dose of streptozotocin. Serum cholesterol level was measured on the days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. *P < 0.05, **P < 0.01 vs. healthy group on each day; ##P < 0.01, ###P < 0.001 vs. diabetic control on each day; +P < 0.05 vs. the day 0 in each group.
Simple effects univariate analysis of groups for each day revealed a significant increase of cholesterol in diabetic control group on the days 17 (P = 0.033) and 31 (P = 0.009) in comparison with healthy rats. When compared to diabetic control group, the cholesterol level of groups receiving asafoetida 50 (P = 0.001) and 100 mg/kg (P = 0.003) was significantly lower on the day 31.
Multivariate simple effect analysis of days for each group revealed no significant change in cholesterol level for healthy rats and groups receiving asafoetida 50 mg/kg and 100 mg/kg (P > 0.05), but a significant change was observed in diabetic controls. In the diabetic control group, multiple comparisons with corrected Bonferroni test showed that cholesterol level significantly increased on the days 17 (P = 0.009) and 31 (P = 0.001) compared with the day 0.
4.3. The Effect of Ethyl Acetate Extract of Asafoetida on Triglyceride
The 3 (time) × 5 (groups) mixed model ANOVA revealed significant main effects of time [F (2, 70) = 23.25, P < 0.001], treatment [F (4, 35) = 7.5, P < 0.001], as well as significant time × treatment interaction [F (8, 70) = 8, P < 0.001] on triglyceride level (Figure 3).
Effect of asafetida extract on the serum triglyceride level (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from the day 3 to 31 after administration of a single dose of streptozotocin. Serum triglyceride level was measured on the days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. ***P < 0.001 vs. healthy group on each day; ##P < 0.01, ###P < 0.001 vs. diabetic control group on each day; +P < 0.05, +++P < 0.001 vs. the day 0 in each group.
Simple effects univariate analysis of groups for each day demonstrated a significant increase of triglyceride in the diabetic control group on the days 17 and 31 compared with healthy rats (P < 0.001). The triglyceride level of groups receiving asafoetida 50 mg/kg and 100 mg/kg significantly decreased on the days 17 (P < 0.01) and 31 (P = 0.001) in comparison with the diabetic group.
Multivariate simple effect analysis of days for each group revealed no significant change in triglyceride level for healthy rats and groups receiving asafoetida 50 mg/kg and 100 mg/kg (P > 0.05), but a significant change was observed in the diabetic control group. Multiple comparisons with corrected Bonferroni test for diabetic control group indicated that triglyceride level significantly increased on the days 17 (P < 0.001) and 31 (P < 0.001) in comparison to the day 0. This indicated that serum triglyceride increased following diabetes induction in animals, and that asafoetida could decrease triglyceride level in a dose and time dependent manner.
4.4. Effect of Ethyl Acetate Extract of Asafoetida on Serum HDL
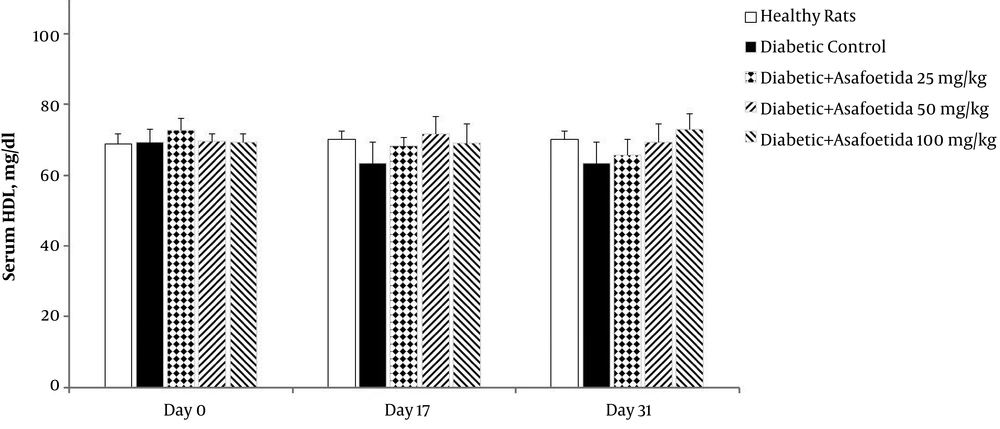
The effect of ethyl acetate extract of asafoetida on serum HDL level in STZ-induced diabetic rats was investigated during the experiment. The 3 (time) × 5 (groups) mixed model ANOVA revealed no significant main effects of time [F (2, 70) = 2.8, P > 0.05[, treatment [F (4, 35) = 0.77, P > 0.05], as well as no significant time × treatment interaction [F (8, 70) = 1.6, P > 0.05] on HDL level (Figure 4). This indicated that diabetes induction and asafoetida treatment had no effect on HDL level of the rats.
Effect of asafetida extract on serum HDL level (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from the day 3 to 31 after administration of a single dose of streptozotocin. Serum HDL level was measured on the days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. There was no significant change in serum HDL level during the experiment.
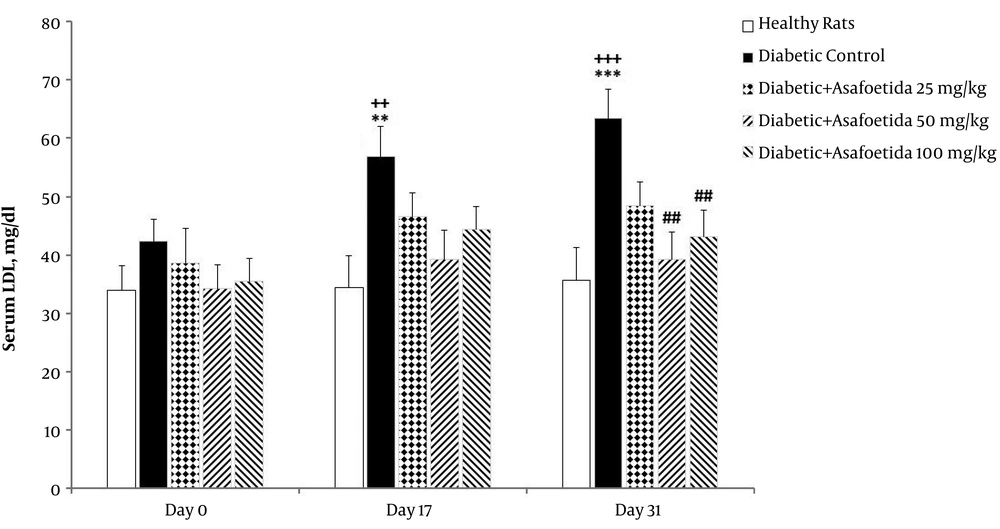
4.5. Effect of Ethyl Acetate Extract of Asafoetida on Serum LDL
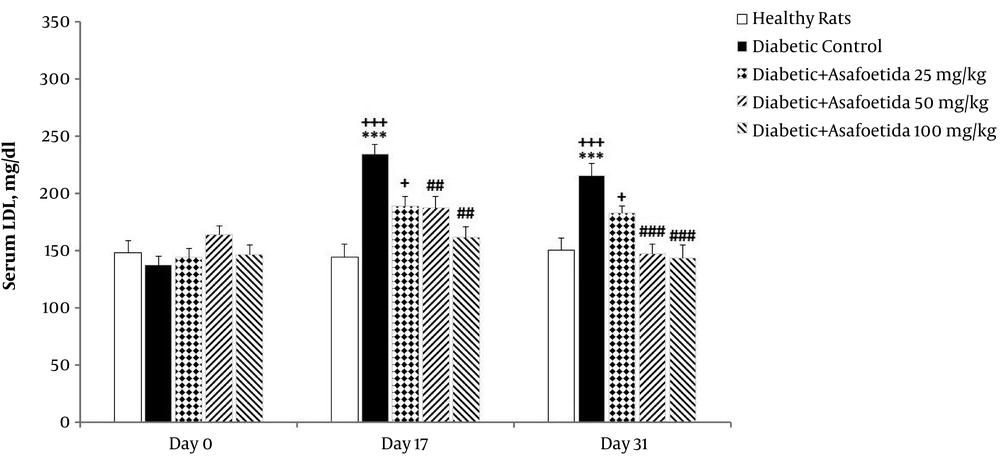
The 3 (time) × 5 (groups) mixed model ANOVA revealed significant main effects of time [F (2, 70) = 7.04, P = 0.002], treatment [F (4, 35) = 5, P = 0.003], and no significant time × treatment interaction [F (8, 70) = 0.92, P > 0.05] on LDL level (Figure 5).
Effect of asafetida extract on serum LDL level (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from day the 3 to 31 after administration of a single dose of streptozotocin. Serum LDL level was measured on the days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. **P < 0.01 and ***P < 0.001 vs. healthy group on each day; ##P < 0.01 vs. diabetic control group on each day; ++P < 0.01 and +++ P < 0.001 vs. the day 0 in each group.
Simple effects univariate analysis of groups for each day revealed significant increase of LDL in the diabetic control group on the days 17 and 31 compared to healthy rats (P = 0.001). LDL was significantly lower in groups receiving asafoetida 50 mg/kg and 100 mg/kg (P = 0.037 and P = 0.007, respectively) than the diabetic control group on the day 31. There was no significant difference between groups on the day 0.
Multivariate simple effect analysis of days for each group showed no significant change in LDL level of healthy rats and groups receiving asafoetida 50 mg/kg and 100 mg/kg (P > 0.05), while a significant change was observed in the diabetic control group.
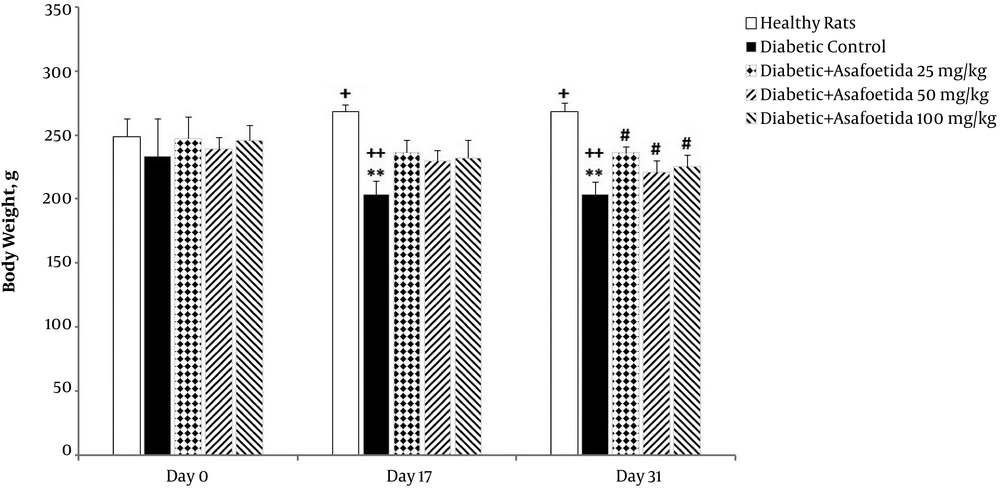
4.6. Effect of Ethyl Acetate Extract of Asafoetida on Animal’s Weight
The 3 (time) × 5 (groups) mixed model ANOVA showed significant main effects of time [F (2, 70) = 7.57, P = 0.001], treatment [F (4, 35) = 9.7, P < 0.001], and also significant time × treatment interaction [F (8, 70) = 4.38, P < 0.001] on animals weight (Figure 6).
Effect of asafetida extract on the animals’ weight in different groups (n = 8); asafoetida extract (25, 50, 100 mg/kg; i.p.) was injected from the day 3 to 31 after administration of a single dose of streptozotocin. Body weight was measured on days 0, 17, and 31. Data were expressed as mean ± SEM and analyzed using the mixed-model ANOVA and Bonferroni post hoc test. **P < 0.01 vs. healthy group on each day; # P < 0.05 vs. diabetic control group on each day; + P < 0.05 and ++ P < 0.01 vs. the day 0 in each group.
Simple effects univariate analysis of groups for each day showed a significant weight loss for the diabetic control group on the days 17 and 31 (P < 0.01) compared to healthy rats. Meanwhile, when compared to the diabetic group, asafoetida receiving groups had a higher weight on the day 31 (P < 0.01).
Multivariate simple effect analysis of days for each group demonstrated a significant increase in weight of healthy rats (P < 0.05), while no significant change was observed in asafoetida receiving groups (P > 0.05). Multiple comparisons with corrected Bonferroni test in the diabetic group showed a significant weight loss on the days 17 (P = 0.003) and 31 (P = 0.001) compared with the day 0.
5. Discussion
The current study aimed at investigating the effect of ethyl acetate extract of F. asafoetida oleo-gum resin on the serum glucose level and lipid profile of STZ-induced diabetic rats. The results showed that diabetes induction could increase serum levels of glucose, cholesterol, LDL, and triglycerides in rats. Moreover, diabetes led to a weight loss in rats. It is shown that streptozotocin injection at doses of 40 to 70 mg/kg can damage pancreatic beta cells of rats and creates a type 1 diabetes mellitus, similar to that of human (21). Streptozotocin can get into pancreatic beta cells by type 2 glucose transporters and increase the production of oxygen free radical species. It seems that oxidative effects, in addition to the direct effect of streptozotocin on the DNA, can induce cell death in beta cells (22). Following the injury or death of beta cells, plasma insulin level reduces, while blood glucose increases. Additionally, it was recently reported that streptozotocin can increase the mRNA expression of ketogenic enzymes in diabetic rats (23), resulting in glucose reduction and shifting the cells to use fatty acids to provide energy. This results in imbalance of fat metabolisms and changes in serum lipid profile.
The current study results revealed that injection of asafoetida extract to diabetic animals could significantly decrease serum glucose. This result was in agreement with that of Abu-Zaiton study, reporting that injection of asafoetida extract for two weeks in alloxan-induced diabetic rats can reduce blood glucose levels and increase insulin secretion (12). In agreement with the current study findings, Iranshahi and Alizadeh demonstrated that injection of aqueous extract of asafoetida at a dose of 50 mg/kg, reduced hyperglycemia in the STZ-induced diabetic rats. They also reported that high doses of the extract (100 mg/k and 300 mg/kg) had no effect on reducing serum glucose level (19). In another study, Jain et al., showed that asafoetida at doses of 100 mg/k and 200 mg/kg could not reduce blood glucose level (24). By comparing the current study findings with those of studies by Iranshahi and Alizadeh and Jain et al., it can be concluded that the higher doses of asafetida extract do not have any glucose lowering effects. Moreover, in the study by Jain, the whole extract of asafoetida was used. Therefore, it may be hypothesized that the presence of some components in the whole extract of asafoetida leads to liver toxicity and inhibition of enzymes involved in the glucose metabolism. Therefore, liver toxicity limits use of the higher doses of this extract in diabetic animals.
Since streptozotocin can increase the amount of reactive oxygen species (25), reinforcing the cellular antioxidant system under such circumstances can protect the beta cells from damage and help them to increase insulin secretion. Phytochemical analysis shows that the asafoetida extract contains the polyphenols such as ferulic acid, tannins, and coumarins (5). Ferulic acid has antioxidant effects and can reduce the production of oxygen free radicals in the beta cells and reduce the streptozotocin toxicity (26, 27).
Polyphenols in the asafoetida extract can inhibit activity of alpha-glycosidase enzyme. Inside gastrointestinal tract, this enzyme converts carbohydrates into absorbable monosaccharides. By inhibiting this enzyme, the glucose absorption decreases, leading to the blood glucose reduction (28). Moreover, ferulic acid in asafoetida extracts increases the activity of liver glucokinase. This enzyme phosphorylates glucose and converts it to glycogen (27). Coumarins and tannins in asafoetida extract, with antioxidant effects, protect pancreatic beta cells against oxidative stress; thereby, increasing insulin secretion from the cells.
In agreement with the findings of the current study, it is shown that asafoetida extract, without damaging the pancreas cells, can decrease blood glucose and increase insulin secretion in diabetic rats (12). Aqueous extract of asafoetida resin can control the sodium-glucose dependent transporter in intestinal epithelial cells and reduce glucose absorption (28). Overall, it seems that the presence of polyphenols, tannins, and coumarins in the ethyl acetate extract of asafoetida, could be partially responsible for antidiabetic effects of asafoetida (29).
Another finding of the current study was that the ethyl acetate extract of asafoetida decreased serum cholesterol and triglycerides levels. These effects may be due to the presence of various flavonoids and terpenoid compounds in the extract (7-10), which by influencing the fat oxidation and excretion of bile cholesterol lower blood cholesterol and triglycerides (30). The last finding of the present study was that the asafoetida extract hindered the animal weight loss. This finding was consistent with previous report by Helal et al. (31).
In summary, ethyl acetate extract of F. asafoetida oleo-gum resin can be useful in reducing serum glucose level and improving lipid profile of diabetic rats. These effects may be due to the antioxidant effects of the extract that protect pancreatic beta cells against oxidative stress; thereby, maintaining insulin secretion, which in turn, reduce serum glucose level and prevent the imbalance of lipid metabolism. Further studies should be conducted on asafoetida extract to recognize its active compounds and clarify the cellular and molecular mechanisms of antidiabetic effects of this extract.