1. Background
Vancomycin (VCM) hydrochloride is a glycopeptide antibiotic that inhibits the biosynthesis of bacterial cell wall peptidoglycan polymer. It is a choice antibiotic for treating resistant infections such as methicillin-resistant S. aureus (MRSA) infections. VCM is mainly excreted via kidneys. One of the major adverse effects of this drug is nephrotoxicity, which has limited its dose and duration of administration (1, 2). Although the exact mechanism of renal toxicity of VCM is not fully understood, some published studies have suggested that the oxidative stress has a crucial role in the pathogenesis of VCM-induced nephrotoxicity (VIN) (3-7). Several antioxidants including: vitamin E (8), 2, 3-dihydroxybenzoic acid (9), curcumin (10) and vitamin C (11) have been reported that reduces VIN. Based on these data, renal tubular ischemia due to the oxidative effects of VCM is the main known mechanism of VIN (5, 12, 13).
Nasturtium officinale R. Br (Watercress) is a perennial plant native to Europe, which has been found in the west and southwestern region of Iran. This plant is freshly used as a vegetable in salads, soups, and other recipes. In traditional medicine, the leaves of the plant are widely used as diuretic, expectorant, blood purifier, hypoglycemic, and odontolgic (14-17). Yazdanparast and colleagues reported that watercress supplementation in diet decreases serum lipids and alters blood antioxidant status in hypercholesterolaemic rats (18). They also reported that it has a potent antioxidant property in vitro and in vitro (18, 19). Our previous study showed that watercress produced a noticeable anti-inflammatory effect in different animals of inflammation (17).
Vitamin E is a potent radical scavenger, which is probably the most important inhibitor of membrane lipid peroxidation. It is a lipophilic agent, which can readily cross cell membranes and exert its effects both intracellularly and in membranes (20). Vitamin E has shown promising effects in gentamicin (21), cisplatin (22), and Adriamycin-induced nephrotoxicity (23).
2. Objectives
Therefore, regarding the role of oxidative stress in VIN and the antioxidant effects of NOE and vitamin E, the objective of this study was to investigate the effect of N. officinale ethanol extract and vitamin E against VIN in rats.
3. Methods
3.1. Drugs and Laboratory Kits
Vancomycin hydrochloride was purchased from Jaber Ebne Hayyan Pharmaceutical Co. (Tehran, Iran.). Trichloroacetic acid (TCA), 1,1,3,3 - tetraethoxypropan (TEP), thiobarbituric acid (TBA), and vitamin E were obtained from Sigma Chemical Co. (Sigma, Germany). Creatinine and urea laboratory kits were purchased from Ziest Chem Diagnostics Co. (Tehran, Iran).
3.2. Plant Material and Extraction
Aerial parts of N. officinale were obtained from the suburbs of Yasuj (kohgiluyeh boyerahmad, Iran) at the end of spring 2014 and was identified by a botanist (department of biology, faculty of Sciences, University of Yasuj). A voucher specimen of it was deposited in the herbarium of biochemistry lab, Faculty of Medicine, Yasuj University of Medical Sciences, Yasuj, Iran. The plant aerial parts were dried in room temperature, protected from direct sunlight, and then powdered. For preparation of hydro-alcoholic extract, the powdered parts of the plant (300 g) were macerated with 2000 mL of EtOH-H2O (7:3) at 45°C for 48 hours. Then the extract was shaken, filtered, and concentrated under reduced pressure in a rotary evaporator at 60°C and dried at room temperature (24). The resulting extract as a percentage of the used dried powder of the plant was approximately 23.6%.
3.3. Determination of Total Phenol
Total phenol content of the extract was determined by Folin-Ciocalteu reagent method (25). An aliquot of 0.1 mL of the extract (1 mg/mL) was combined with 0.4 mL of sodium carbonate 7.5% and 0.5 mL Folin-Ciocalteu reagent. After 30 minutes of being maintained in laboratory temperature, its light absorption was read by spectrophotometer (Farmacia LKB model Neova spect II made in England) in wavelength of 756 nm. Experiments were performed in triplicate. Total phenolic was expressed as milligrams of gallic acid equivalents (GAE) per gram of sample using the standard calibration.
3.4. Determination of Total Flavonoids
The total flavonoids contents of the extract were estimated by aluminium chloride colorimetric method (26). An aliquot of 0.1 mL of the NOE (1 mg/mL) was added to sodium nitrate sodium nitrate (5%). Then 10% of aluminium chloride solution was added to the test tube. After incubation for 5 minutes in a dark place, 1 M NaOH (0.5 mL) was added. The absorbance of the sample was read in a fixed wavelength 510 nm. Experiments were performed in triplicate. The total flavonoid content was calculated from a calibration curve, and the result was expressed as mg rutin equivalent per g extract weight.
3.5. Experimental Animals
A total of 36 male wistar rats, weighting 250 to 350 g, were obtained from the Pasteur Institute of Iran, Tehran, Iran. The animals were fed with rat chaw and tap water ad libitum and acclimatized under a temperature of 21 ± 3°C with a 12 hours light/12 hours dark cycle. The experiments were carried out in accordance to the international guidelines for the care and use of experimental animals and approved by the ethical committee of the AJA University of Medical Sciences.
3.6. Experimental Protocol
This dose of VCM was selected based on previous studies (4, 6, 8, 9). The rats were weighed prior to the first injection of VCM and every other day in order to adjust the dose according to weight changes. VCM and NOE were dissolved in normal saline (N/S) and prepared freshly every day. The vitamin E solution was prepared in sesame oil. Vitamin E (250, 500 mg kg-1) or NOE (250 and 500 mg kg-1) administrated orally (p.o.) 30 minutes prior to injection of VCM.
The animals were divided into 6 experimental groups as follows:
Control group: rats received a daily injection of 0.5 mL N/S for 7 days
VCM group: rats received VCM (200 mg kg-1 i.p.) twice a day for 7 days
VCM + NOE group: rats received VCM (200 mg kg-1i.p.) and NOE (250 mg kg-1 p.o.) twice a day for 7 days
VCM + NOE group: rats received VCM (200 mg kg-1 i.p.) and NOE (500 mg kg-1 p.o.) twice a day for 7 days
VCM + vitamin E group (250 mg kg-1, p.o.): rats received VCM (200 mg kg-1 i.p.) and vitamin E group (250 mg kg-1 p.o.) twice a day for 7 days
VCM + vitamin E group: rats received VCM (200 mg kg-1 i.p.) and vitamin E group (500 mg kg-1 p.o.) twice a day for 7 days
3.7. Sample Collection
After the 14th injection of VCM, the animals, while they had free access to tap water, placed individually in propylene metabolic cages at 8 p.m. and the 12 hours urine was collected and urine volume was measured. Blood samples were collected by cardiac puncture from all the animals under ether anesthesia. The blood and urine samples were centrifuged at 3000 rpm for 20 minutes at +4°. The supernatant was separated and stored at -20°C until analyzed. After collecting the blood specimens, the animals were sacrificed by diethyl ether and both kidneys were removed and weighed. The right kidneys were prepared for pathologic examinations and the left kidneys stored at -80°C for MDA measurement.
3.8. Biochemical Assays
Urea and creatinine levels were determined according to commercial kits instructions (Ziest Chem Diagnostics Co., Iran).
3.9. Measurement of Creatinine Clearance
The 12 hours urine volume was determined (Uvol in mL) and the creatinine concentration (Ucr in mg/dL) of it was measured by using the Jaffe method. In addition, serum creatinine (Pcr in mg/dL) concentrations were measured. The time span of the urine collection was calculated in minutes (12 hours = 720 minutes). The creatinine clearance (Clcr) is calculated with the following formula (27, 28):

3.10. Measurement of Renal Lipid Peroxidation
The concentrations of kidney MDA as proceeding of lipid peroxidation were determined according to a modified method of Ohkawa (29). The kidney tissues were homogenized in a Teflon glass homogenizer with a normal saline (NS) to obtain a 10% (w/v) kidney homogenate. Homogenates were centrifuged at 18,000 × g (+ 4°C) for 30 minutes. A total of 0.5 mL of kidney homogenate solutions was mixed with 2 mL of the TBA-TCA reagent (0.375% w/v TBA, 15% w/v TCA, 0.25 N HCl). The mixture was heated for 15 minutes in a boiling water bath. Next, it was cooled in a cold water bath for 10 minutes and centrifuged at 2000 × g for 15 minutes. The absorbance of solution was measured spectrophotometrically at 532 nm with respect to the blank solution and it was expressed as µmol/gram wet tissue.
3.11. Histopathologic Examination
The right kidney was excised after sacrifice, halved, and fixed by immersion in 10% formaldehyde solution for several days. After that, the fixed tissues were embedded in paraffin and cut into 4 - 5 μm slices. The slices were mounted on the glass slides and stained with hematoxylin and eosin (H and E) for light microscopy analysis. The assessment was conducted by a pathologist in a blinded way.
3.12. Statistical Analysis
All results are expressed as mean ± S.E.M. The differences between the control and treatment groups were tested by one-way analyses of variance (ANOVA) followed by the Tukey post-hoc test, using the SPSS 19 for windows. P values less than 0.05 were considered to show significant differences for all comparisons made.
4. Results
4.1. Total Phenolic and Flavonoid Contents
The overall mass yield of the extract from the plant material was 23.6%. Phenolic and flavonoid contents of N. officinale extract find out to be 78 ± 6.32 gallic acid equivalents per gram dried extract (mg GAE/g) and 96.46 ± 8.11 mg rutin equivalents per gram dried extract (mg RE/g), respectively.
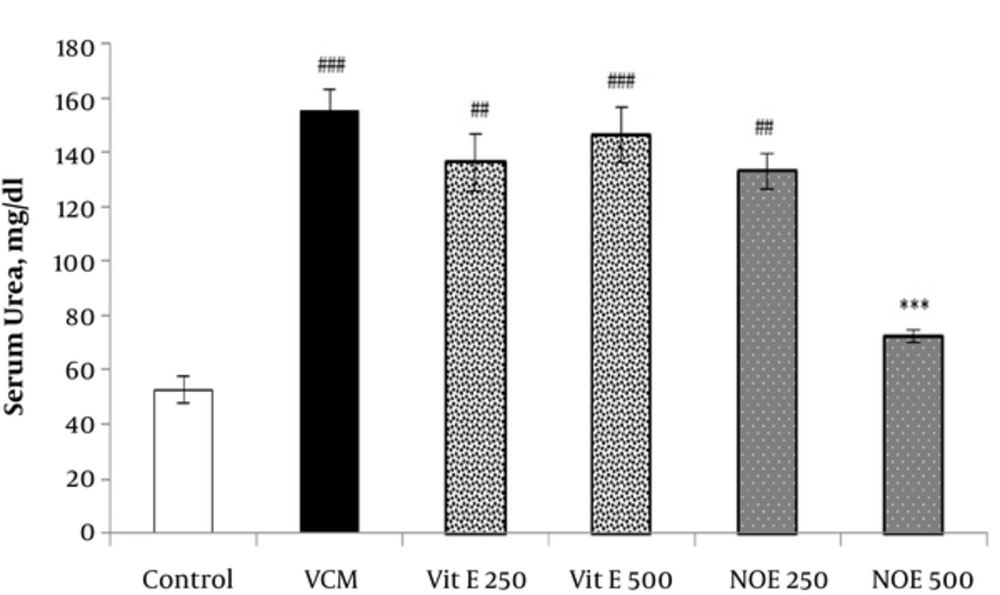
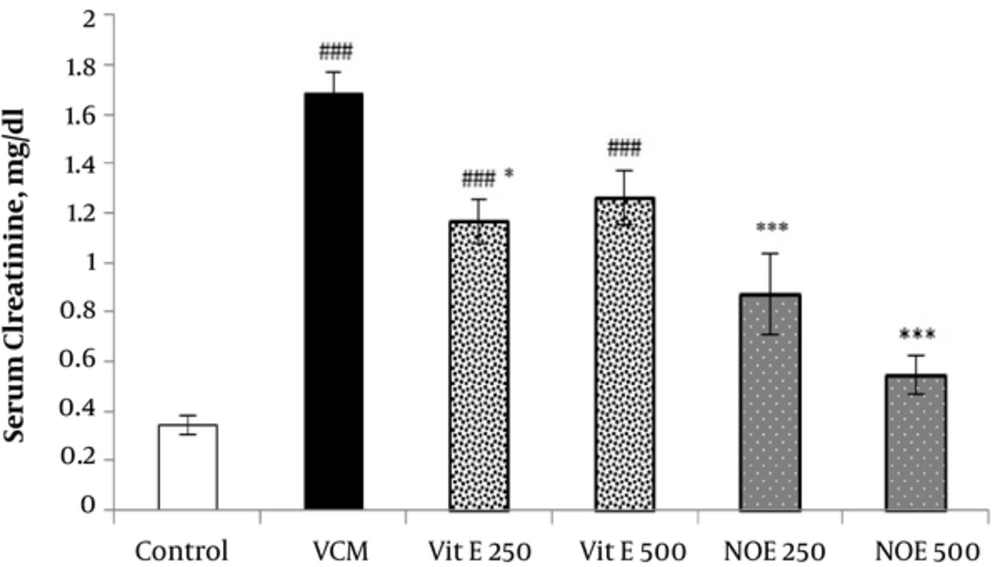
4.2. Effect of NOE and Vitamin E on VCM-Induced Changes in Serum Creatinine and Urea Concentrations
As shown in Figures 1 and 2, injection of VCM (200 mg kg-1) for 7 consecutive days significantly increased the serum creatinine and urea compared to the control groups (P < 0.001). Oral pre-treatment with 250 and 500 mg kg-1 NOE and 500 mg kg-1 vitamin E considerably prevented vancomycin-induced increase in creatinine levels compared to the VCM group (P < 0.001). NOE 500 mg kg-1 also significantly inhibited the elevation in serum urea (P < 0.001). Vitamin E at dose of 250 mg kg-1 did not modify the vancomycin-induced changes in urea and creatinine levels.
All test groups received the indicated dose of Vit E or NOE plus 200 mg kg-1 VAN. The control group received only normal saline. Values are mean ± S.E.M. VCM, vancomycin; NOE, N. officinale extract; (n = 6) ##P < 0.01, ###P < 0.001 vs. normal saline group (I); ***P < 0.001 vs. VCM group (II).
All test groups received the indicated dose of Vit E or NOE plus 200 mg kg-1 VAN. The control group received only normal saline. Values are mean  ± S.E.M. VCM, Vancomycin; NOE; N. officinale extract; (n = 6) #P < 0.05, ##P < 0.01, ###P < 0.001 vs. normal saline group (I); *P < 0.05, **P < 0.01, ***P < 0.001 vs. VCM group (II).
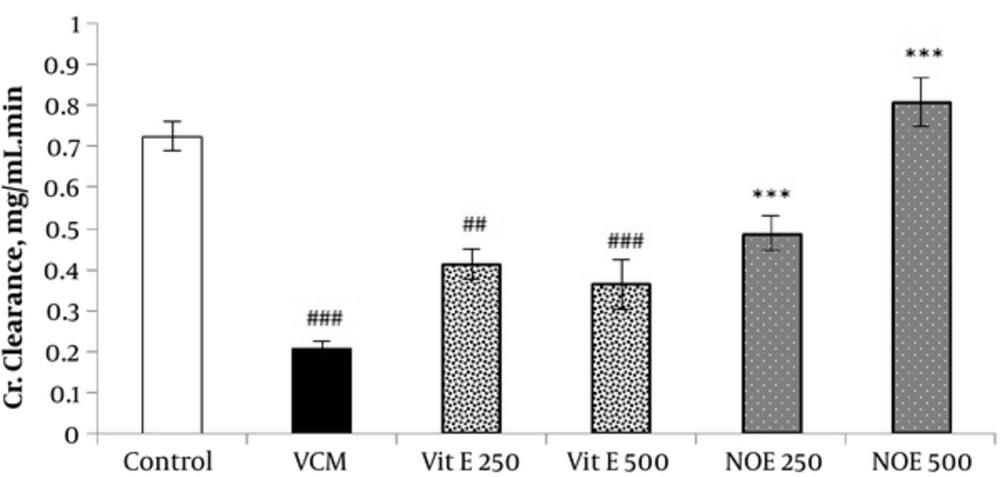
4.3. Effect of NOE and Vitamin E on VCM-Induced Changes in on Creatinine Clearance
VCM injection (200 mg kg-1) for 7 consecutive days induced a noticeable decrease in creatinine clearance compared to control groups (P < 0.001). Oral treatment with NOE and vitamin E at doses of 250 and 500 mg kg-1 improved the creatinine clearance compared to the VCM group (P < 0.001).
4.4. Effect of NOE and Vitamin E on VCM-Induced Changes in Renal MDA Level
As shown in Table 1, kidney MDA content, an indicator of lipid peroxidation, was increase in VCM-treated rats compared to control groups (P < 0.001). NOE and vitamin E at the all indicated doses inhibited elevation in the kidney MDA levels due to VCM injection (P < 0.001).
| Treatment Groups | Percent Change in Body Weight | Kidney Weight, g/100 g body weight | Urine Volume, mL/12 h | MDA, µmol/ gram tissue |
|---|---|---|---|---|
| I- Control | +1.30 ± 0.51 | 0.39 ± 0.01 | 6.88 ± 0.83 | 1.89 ± 0.32 |
| II. VCM + Vit E 250 | -10.2 ± 0.85b | 0.92 ± 0.04b | 4.45 ± 0.48 | 7.83 ± 0.75b |
| III. VCM + Vit E 250 | -6.30 ± 1.30c,d,e | 0.61 ± 0.10f | 7.80 ± 0.48 | 4.48 ± 0.55g |
| IV. VCM + Vit E 500 | -1.30 ± 0.77g | 0.67 ± 0.01f,h | 9.50 ± 1.43f | 2.54 ± 0.53g |
| V. VCM + NOE 250 | -5.51 ± 1.29d,f | 0.78 ± 0.04b,e | 9.58 ± 1.32g | 3.85 ± 0.52g |
| VI. VCM + NOE 500 | -1.40 ± 0.59g | 0.57 ± 0.03g | 11.6 ± 0.76g,h | 1.68 ± 0.15g |
Abbreviations: NOE, N. officinale Extract; VCM, Vancomycin.
aValues are mean ± S.E.M. (n = 6).
bP < 0.001 vs. normal saline group (I).
cP < 0.05.
dP < 0.01.
eP < 0.05 vs. group (VI).
fP < 0.01.
gP < 0.001 vs. VCM group (II).
hP < 0.05.
4.5. Effect of NOE and Vitamin E on VCM-Induced Changes in Body and Kidney Weight
As illustrated in Table 1, i.p. injection of VCM (200 mg kg-1) decreased the animal’s weight significantly during treatment course compared to the initial weight (41.8 percent). The decline in body weight was markedly inhibited by pre-treatment with NOE 500 mg kg-1 and vitamin E 500 mg kg-1 (P < 0.001).
The ratio of average weight of left and right kidneys to 100 g body weight also significantly increased in the VCM-treated animals compared to the control group (P < 0.001, Table 1). Both NOE 500 mg kg-1 and vitamin E 500 mg kg-1 considerably reduced this change (P < 0.01 and P < 0.05, respectively).
4.6. Effect of NOE and Vitamin E on VCM-Induced Changes in Urine Volume
VCM treated animals characterized by having a considerably lower urine volume when compared with the control rats. This change was significantly reversed in the vitamin E (500 mg kg-1) and NOE (250 and 500 mg kg-1) groups. However, the changes of urine volume did not significantly inhibited at a lower dose of vitamin E (250 mg kg-1).
4.7. Pathological Examination
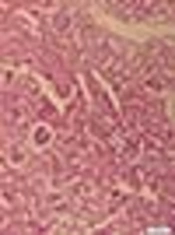
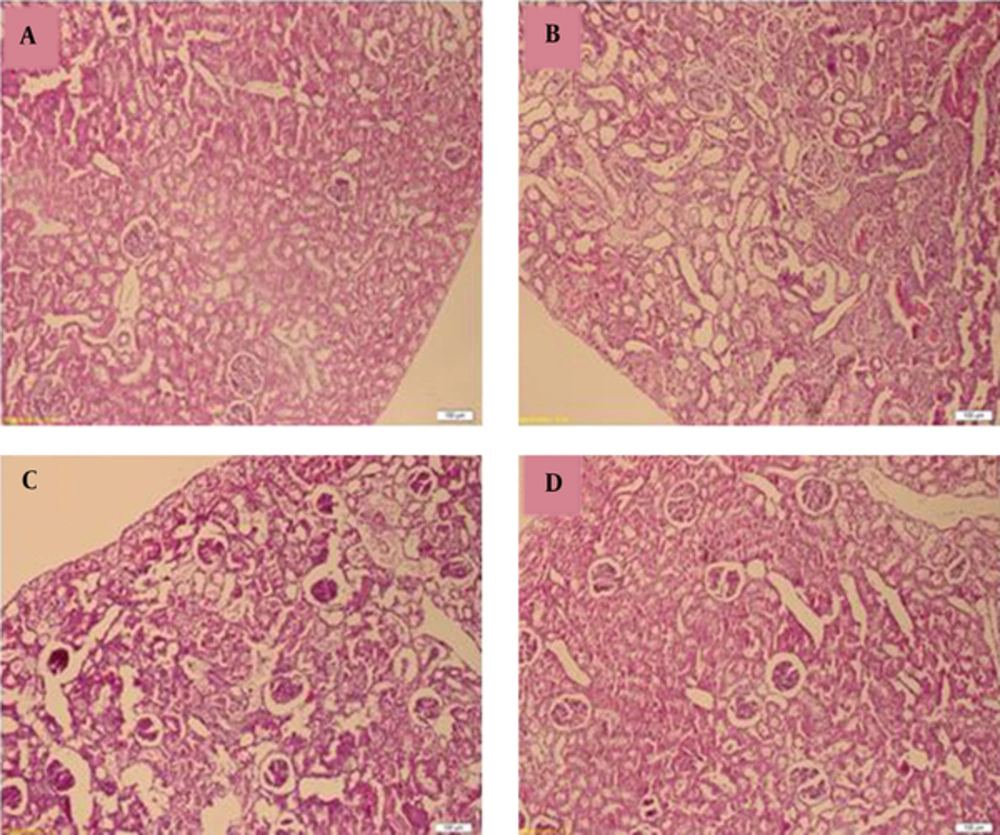
Figure 4 represented the histological micrographs of renal from the 4 groups used. Histopathological evaluation of VCM-treated rat kidney showed renal parenchymal damage, such as interstitial swelling, tubular dilatation, tubular epithelial cell desquamation. These histopathological changes observed were considerably inhibited by NOE and vitamin E.
All test groups received the indicated dose of Vit E or NOE plus 200 mg kg-1 VAN. The control group received only normal saline. Values are mean  ± S.E.M. VCM, Vancomycin; NOE, N. officinale extract; (n = 6), ##P < 0.001, ###P < 0.001 vs. normal saline g roup (I); ***P < 0.001 vs. VCM group (II).
A, Control (renal tubules are normal). B, vancomycin alone (Tubules show extensive and marked parenchymal damage, such as interstitial swelling, tubular dilatation and Neutrophil (PMN) infiltration. C, Simultaneous treatment with vancomycin and vitamin E; D, Simultaneous treatment with vancomycin and Nasturtium officinale. Administration of Vitamin E and Nasturtium officinale reduced the severity of the damage in the proximal renal tubules. (H and E).
5. Discussion
Renal toxicity is one of the most important side effects of VCM, therefore their clinical uses are limited (1, 2). Numerous therapeutic agents have been investigated clinically and experimentally against this adverse effect, however, none of them confirmed to be clinically effective as a complete protective component (8-11). In the present study, repeated i.p. administration of 200 mg kg-1 VCM, for 7 consecutive days to rats, induced a considerable raise in serum creatinine and urea concentrations compared to the control group, suggesting an acute renal failure. These findings are in good agreement with the results of a previous study about the nephrotoxicity of VCM in experimental animals (4-6). Naghibi and colleagues reported that i.p. injection of VCM at dose of 200 mg kg-1 elevated these parameters in rats (8, 9).
In the first stage of renal diseases, serum creatinine level is an important indicator than the urea. Serum urea concentrations start to elevate only after kidney parenchymal damage (30). Oral administration of NOE (250 and 500 mg kg-1) and 500 mg kg-1 vitamin E reversed the increase in serum creatinine levels due to VCN injection. To our knowledge, there is no report on the nephrophrotective effects of N. officinale in different models of renal injury. Similar inhibitory effect on serum creatinine concentration suggested about vitamine E and other antioxidant in the VIN (8-11). Kadkhodaee and et al. observed that co-administration of moderate doses of vitamins C and E had beneficial effects on renal preservation in GM-induced nephrotoxicity in rat (31). The increased levels of plasma urea, because of VCM injection, also significantly reduced by NOE at 500 mg kg-1 dose, however, vitamin E reduced plasma levels approximately 6% - 10%. This finding regarding vitamin E to some extent is inconsistent to what has been reported by Naghibi et al., (8). One possible explanation for these different results might be hidden in the fact that bioavailability of vitamin E in parenteral route is more than their oral intake (32). Therefore, in our experimental condition (oral administration), it is possible that vitamin E did not reach sufficient plasma levels to show his reducing effect on the plasma urea levels. It is important to mention that NOE and vitamin E efficiently prevented VCM induced decrease in creatinine clearance.
Changes in body and kidney weights are valuable indicators of severe renal toxicity in the experimental models (6, 8, 9, 33). In our experimental condition, VCM caused a significant reduction in the body weight and increased the ratio of average kidneys to 100 g body weight. Pretreatment with NOE and vitamin E revered the indicated changes at the applied dose, as their effects were statistically significant at the highest dose. In agreement with this finding Naghibi et al., observed that subcutaneous injection of vitamin E significantly reduced the changes in body and kidney weights due to VCM toxicity (8, 9).
The precise mechanism VCM-induced renal toxicity is not very clear, however, the role of oxidative stress in the pathogenesis of VCM-induced nephrotoxicity has been suggested by many investigators (3-7). Reactive oxygen species (ROS) induce lipid peroxidation through interaction with the membrane bound polyunsaturated fatty acids. MDA is the final metabolite of lipid peroxidation chain; it indicates an increase of free radical generation (34). In the present study, the values of kidney MDA considerably increased in VCM-treated rats. Oral administration of NOE and vitamin E were able to inhibit the increase in the kidney MDA levels as a result of VCM toxicity. Several investigations suggested antioxidant properties of N. officinale. Watercress ingestion exhibited noticeable antioxidant effects against exercise-induced DNA damage and lipid peroxidation (35). Water and ethanol extracts of watercress also inhibited lipid peroxidation in linoleic acid, liver, brain, and kidney homogenate model systems (36). In our earlier study we found that NOE exhibited a noticeable anti-inflammatory activity in several animal models of inflammation (17). Moreover, free radical scavenging properties of phenolic and flavonoid compounds were confirmed in some studies (37-39). In this study, phenolic and flavonoid contents of NOE detected to be 71 ± 6.23 gallic acid equivalents/g dried extract and 95.26 ± 7.6 mg rutin equivalents/g dried extract, respectively. Based on this, it is likely that beneficial effects of N. officinal,e in reducing of VCM renal toxicity, were mediated through antioxidant pathway. Histological observation confirmed the biochemical finding. Biopsies from the kidney of VCM-treated rats showed a marked renal injury with tubular epithelial cell desquamation, swelling, and tubular dilatation. Histopathological changes were significantly amiliorated by NOE- and vitamin E administration.
In conclusion, this study is the first scientific demonstration that proposes renoprotective potential of NOE and also confirms its traditional use as a treatment for urinary disorders. Further studies are needed to recognize and purify the active phyto-ingredients from NOE that are important in its nephroprotective effect.