1. Background
Today, Schiff base coordination chemistry has raised the interest of medicinal chemists and is extensively applied in medicine and industry. The importance of Schiff base complexes to bioinorganic chemistry, biomedical programs, supramolecular chemistry, and formation of compounds with unusual characteristics are nicely identified and reviewed. Schiff base and its complexes found a reflective and imperative place in both organic and inorganic chemistry. They represent a versatile class of compounds gifted with vast choice of biological applications (1). They are synthesized without any difficulty, are easily available, and display various denticities and functionalities. Schiff base metal complexes encompass a huge area of research due to their possible importance in various fields of chemistry including medicinal chemistry, bioinorganic chemistry, etc. (2-4). The ligands and their metal complexes play outstanding role as preparatory materials in different industries (5, 6). The abovementioned advantages made these compounds as the proficient candidates to raise the attention of researchers toward synthesizing new metal complexes applicable to bioinorganic chemistry, homogenous or heterogeneous catalysis, encapsulation, transport, and separation processes (7). In such compounds the iminic (-C = N-) nitrogen are the important coordinators and also have specific biological applications (8). Several complexes of the Schiff base ligands possess anticancer (9), antifungal, antibacterial, and herbicidal activities (10). The distinctive nature of such compounds makes them potential antimicrobial agents with the ability to be combined with lipid layer, and hence, favour the permeation of complexes via the phospholipid membrane.
The current study aimed at preparing, spectrally analysing, and biologically evaluating a unique organic ligand and its mononuclear Cu (II) and Zn (II) complexes synthesized by the reaction of 1, 2-diphenylethane-1, 2-dione and dinitrophenyl hydrazine. The obtained ligand and its copper (II) and zinc (II) complexes were screened in vitro against different types of bacteria and fungi to assess their antimicrobial potentials.
2. Methods
2.1. Materials and Methods
The analytical grades of 1, 2-diphenylethane-1, 2-dione and dinitrophenyl hydrazine were purchased from Sigma Aldrich Chemicals Pvt. Ltd. Bangalore, India and used without any further purification. The metal salts, copper chloride and zinc chloride, were supplied by Sd Fine Chemicals Limited Mumbai, India. C, H, and N analysis was performed with a Perkin–Elmer 2400 series II analyzer (Perkin Elmer, Massachusetts, USA). 1H-NMR (proton NMR, hydrogen-1 NMR) spectroscopy of the synthesized compounds was performed on a Varian 400 MHz spectrometer (Avance III, 400 MHz Bruker, Massachusetts, USA) in d-CDCl3, using TMS (SiMe4) as an internal reference at room temperature. The FTIR (Fourier-transform infrared spectroscopy) of the desired compounds were collected on a Tensor II FTIR Spectrometer (Bruker, Massachusetts, USA) from 4000 - 400 cm-1 at 25°C using KBr plates. The ultraviolet-visible spectroscopy (UV-Vis) of the synthesized compounds was recorded in dimethyl sulfoxide (DMSO) 1 × 10-4 M and a quartz glass cell with a path length of 10 mm on a Lambda 40 spectrophotometer (Perkin Elmer, Massachusetts, USA). X-ray diffraction of the ligand and its copper and zinc complexes were carried out with powder compounds using Rigaku Ultima IV (Tokyo, Japan) Diffractometer. The molar conductance values (10-3 M/L) of the complexes, in DMSO, were measured utilising a Systronic Conductivity Bridge 304 (Systronics India Ltd. Gujarat).
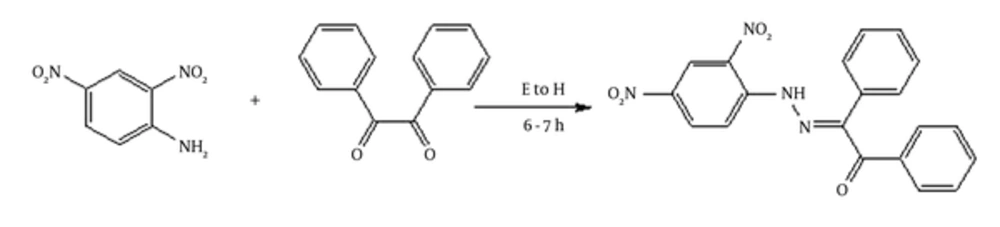
2.2. Synthesis of Schiff Base (E)-2-(2-(2,4-Dinitrophenylhydrazono)-1,2-Diphenylethanone
Hot ethanoic solutions (20 mL) of 1,2-diphenylethane-1, 2-dione (1 g, 5 mM) and dinitrophenyl hydrazine (0.93 g, 5 mM) were charged in a 250-mL flask with continuous stirring. The reaction was followed by refluxing in a water bath at 75°C for 6 - 7 hours by adding the catalytic amount of concentrated HCl (pH 3 - 4). The completion of the reaction was checked by taking a thin-layer chromatography (TLC) at appropriate intervals. On the completion of reaction, a yellow coloured precipitate was appeared and then, filtered and washed with cold EtOH, and dried under vacuum (Figure 1).
Yield: 67%. Anal. Calc. (%) for C20H14N4O5 (390 g/M): C, 61.54; H 3.62; N, 14.33. Found: C, 61.50; ν H, 3.58; N, 14.29. FT-IR (KBr), cm-1: ν (N-H) 3336, (C = N) 1625, ν (C = O) 1710, ν (NO) 1610.1H-NMR (d-CDCl3): 9.01 (s, NH); 7.18 - 8.76 (m, Ar-H) ppm. UV/Vis (DMSO, λmax (ε, L/M-cm): 40,816, 32,258. MS (m/z): 391 [M + H]+
2.3. Synthesis of Metal Complexes
Complexation of synthesized ligand was achieved by adding 25 mL of ethanoic solution of zinc chloride or copper chloride (5 mM) to a stirring ethanoic solution (5 mM, 25 mL) of ligand charged in a round bottom flask. The reaction was carried out by maintaining the metal/ligand ratio of 1:2. The reaction was refluxed for 2 hours in a water bath till the appearance of precipitate. TLC was continuously taken to check the progress of reaction. The formed precipitate was collected by filtration, and washed several times with distilled water and cold EtOH. The product was then dried in a vacuum desiccator (Figure 2).
[Cu(L)]: Yield: 72%. Anal. Calc. (%) for (C40H28CuN8O10) (844.24 g/M): C, 56.91; H 3.34; N, 13.27. Found: C, 56.88; H 3.29; N, 13.21. FT-IR (KBr), cm-1: ν (N-H) 3342, ν (C = N) 1610, ν (C = O) 1710, ν (NO) 1610, ν (M-N) 446. 1H-NMR (d-CDCl3): 9.0 (s, 1H); 7.10 - 8.80 (m, Ar-H) ppm. UV/Vis (DMSO, λmax (ε, L/M-cm): 25, 626, 10, 350. MS (m/z): 845.12 [M + H]+
[Zn(L)]: Yield: 69%. Anal. Calc. (%) for (C40H28ZnN8O10): (846 g/M): C, 56.78; H 3.34; N, 13.24. Found: C, 56.72; H, 3.28; N, 13.20. FT-IR (KBr), cm-1: ν (N-H) 3343, ν (C = N) 1620, ν (C = O) 1705, ν (NO) 1610, ν (M-N) 435. 1H-NMR (d-CDCl3): 9.0 (s, 1H); 7.10 - 8.75 (m, Ar-H) ppm. UV/Vis (DMSO, λmax (ε, L/M-cm): 40,000. MS (m/z): 845 [M]+
2.4. Antimicrobial Activity
The synthesised ligand and its metal complexes were evaluated for their antimicrobial potential against some strains of bacteria and fungi such as Bacillus subtilis, Staphylococcus aureus, Escherchia coli, Candida albicans, Aspergillus flavus, and Aspergillus niger. The testing solutions were prepared by dissolving the synthesized compounds in DMSO.
3. Results and Discussion
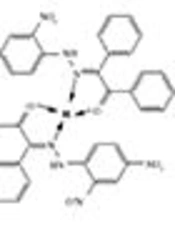
All complexes gave satisfactory elemental measures, conformed to those proposed by the suggested formulae (supplementary file Appendix 1). The metal complexes were completely miscible in DMSO and DMF, but insoluble in nonpolar organic solvents and water. The molar conductance values measured by Systronic Conductivity Bridge 304 and indicated that the complexes were naturally non-electrolyte (11). Despite the authors’ exhaustive efforts, the study failed to get single crystals of the complexes for carrying out X-ray crystallographic assessments. However, the possible structure of the synthesized complexes was predicted based on analytical, spectroscopic, and magnetic data.
3.1. Molar conductance and elemental analysis
The molar conductivity of the synthesised metal complexes in DMF solution (10 - 3 M) ranged 12 - 18 Ω/M-cm2 indicating the non-electrolytic nature of the metal complexes and the coordination of chloride anion with the central metal ion (12, 13). The results obtained from spectroscopic evaluations of the metal complexes were in close agreement with the calculated values, suggesting that the complexes had 1:2 metal/ligand ratios.
Molar conductance and elemental analysis data (supplementary file Appendix 1) confirmed the suggested formula for metal complexes as [M (C20H14N4O5)] where M = Cu (II) and Zn (II).
3.2. Infrared Spectra
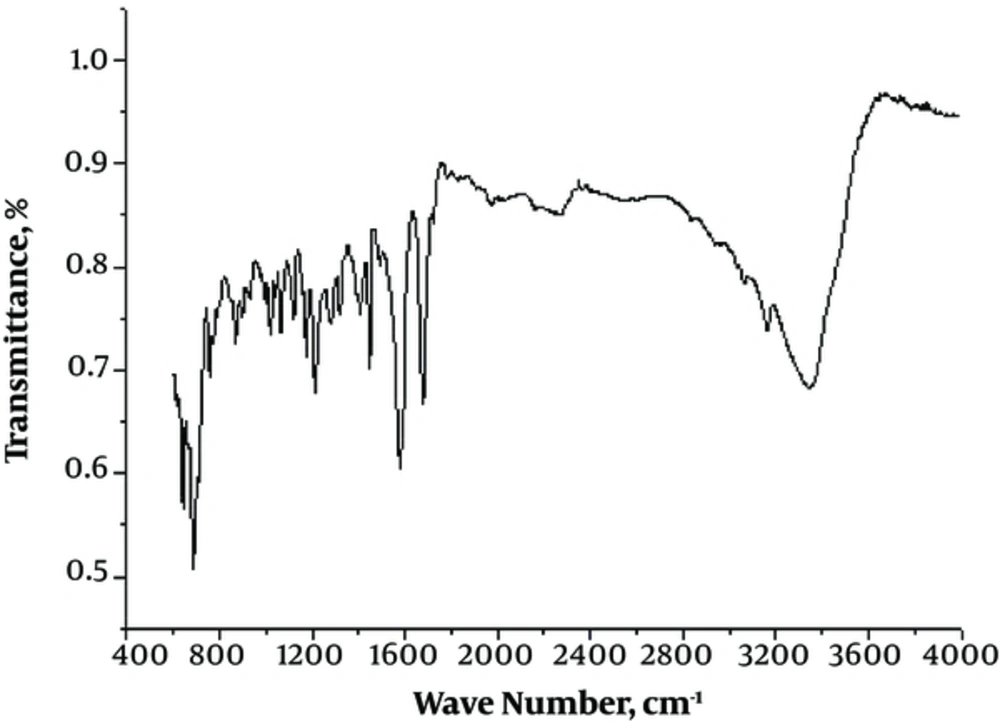
The IR spectra (range 4000 - 200 cm-1) of both the ligand and its complexes conformed to the basic characteristic of the expected peaks. The IR spectrum of the synthesized ligand (Figure 3) showed an absorption peak at 1,625 cm-1 that corresponded to the imine (> C = N) group (14); also a sharp peak at 1,710 cm-1 corresponded to the carbonyl (> C = O) group, which confirmed the complete condensation of the 2 reacting moieties. In addition to these peaks, absorption bands observed in 3340 - 3330 cm-1 and 1500 - 1630 cm-1 regions were assigned to the -NH and -NO groups presented in the ligand. Although, based on the complexation analysis, a strong peak at 1625 cm-1 slightly shifted toward lower frequencies by 15 - 5 cm-1, it confirmed coordination through the iminic nitrogen (> C = N). Another peak within the range of 460 - 420 cm-1 was also ascribed to metal nitrogen (M-N) bonds (15). Hence, based on the IR spectral analysis it was clear that the synthesized ligand behaves as a bidentate coordinating ligand with Cu (II), Co (II), and Ni (II) in 2:1 ratios. The spectral data conformed to the main spectral signals of the Schiff base ligand and its metal (II) complexes are given in the supplementary file Appendix 2.
3.3. Mass Spectra
The synthesized ligand and its metal complexes were studied with electrospray ionization (ESI) mass spectra. The spectrum of the ligand (supplementary file Appendix 3) showed a distinct molecular ion peak at m/z = 391 amu (atomic mass unit), which confirms the molecular mass and suggested structure of the ligand. In the mass spectra, the complexes displayed molecular ion peak [M]+, m/z at 845.12 and 845 amu showing good agreement with their molecular formulae [Cu(C40H28N8O10)] and [Zn(C40H28N8O10)], respectively. The data obtained from the mass spectroscopy confirms the proposed structures and molecular formulae satisfactorily. There were various other peaks in the spectra due to the thermal dissociation of the complexes assigned to various fragments of the dissociated molecule.
3.4. 1H-NMR
1H-NMR spectroscopy is the main technique used to predict the structure of the synthesized compounds. The 1H-NMR spectral data of the ligand and its metal complexes were recorded in chloroform solution with tetramethylsilane (TMS) as an internal standard (supplementary file Appendix 4). In the 1H-NMR spectrum, all the metal complexes showed a multiplet region in 9.80 corresponding to the secondary amide proton (C-NH) (16). The aromatic protons of the ligand were observed as a multiplet compounds at 7.18 - 8.76 ppm (17, 18).
3.5. Electronic Spectral
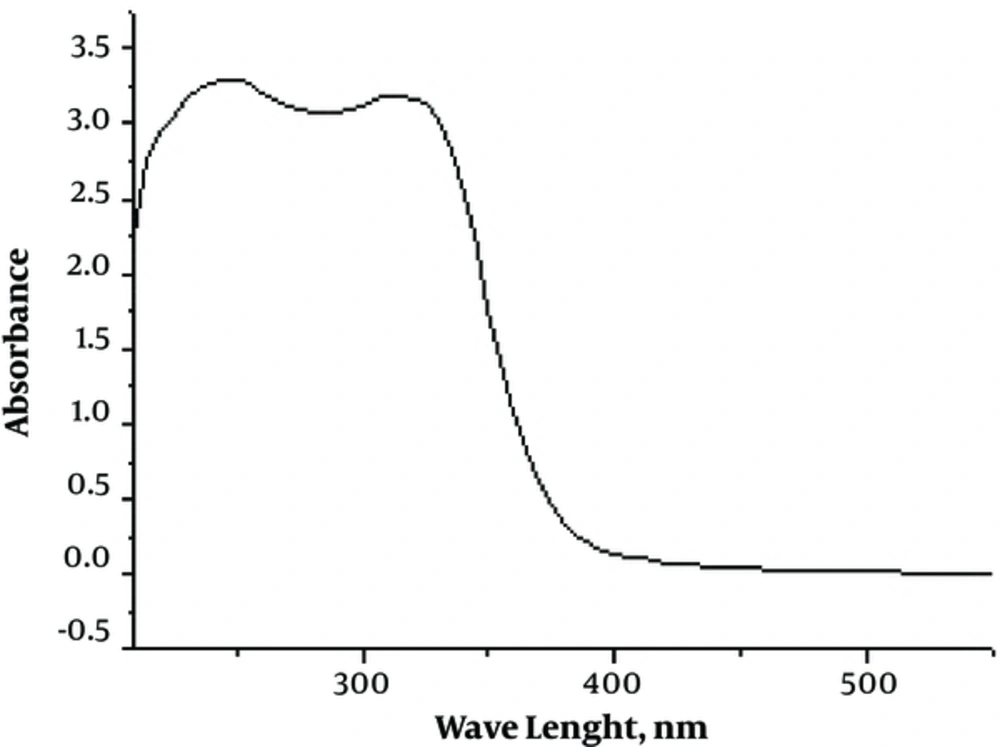
The electronic spectra of Schiff base ligand and Cu (II) and Zn (II) complexes were recorded at room temperature in DMSO-d6. The transitions and assignments are given in in supplementary file Appendix 5. The electronic spectrum of the ligand recorded in a 10-4 M DMSO solution displayed 2 broad bands at 245 and 310 nm (Figure 4). The transition at 245 nm corresponds to the π - π* transition within the aromatic ring of ligand and the band at 310 due to the π - π* transitions of the > C = N- group. This band shifts slightly toward the higher energy region in the spectra of metal complexes due to the polarization within the > C = N chromophore caused by the metal-ligand electron interaction (19). A charge transfer band was exhibited by the Cu (II) complex at 25,626 cm-1. The d-d transition at 10,350 cm-1 of low intensity indicated that the complex exhibits a distorted tetrahedral geometry as a result of the Jahn-Teller distortion. Due to the variation of Cu (II) complex structure from planar to distorted tetrahedral geometry, there is a lowering of d-d transition energy as per crystal field theory. According to the crystal field theory, the d-d transition absorption band envelope shifts toward lower energy as the planar Cu (II) complex twisted towards the distorted tetrahedral structure (20). No d-d electronic transition was observed in Zinc (II) complex because of its fully filled d-orbital. Thus, it seems that the zinc (II) complexes, in general, have a tetrahedral geometry.
3.6. X-Ray Powder Diffraction
X-ray diffraction is an essential instrumental technique utilized to detect crystalline materials. X-ray diffraction of the ligand and its copper and zinc complexes were carried out with powdered compounds using Rigaku Ultima IV X-ray diffractometer at 25°C in Cu anode material and the generator settings of 30 mA, 40 kV. The compounds were recorded at 2θ = 10 - 80°. X-Ray diffractograms designates the crystallinity of the compounds. The studied compounds showed a good quality diffractograms and all the peaks were identified for h, k, and l values using methods reported in the literature. The Braggs equation nλ = 2dsinθ was used to obtain the d values. The h, k, and l values and the cell parameters were used to calculate the values of sin 2θ for each peak. The lattice constants a, b, and c for each unit cell were measured (Figures 5 and 6). Based on X-ray diffraction findings, the copper and zinc complexes were naturally crystalline with monoclinic and orthorhombic crystal systems, respectively. All the peaks were matched by the Joint Committee on Powder Diffraction Standards (JCPDS).
3.7. Biological Activity
3.7.1. Antimicrobial Activity
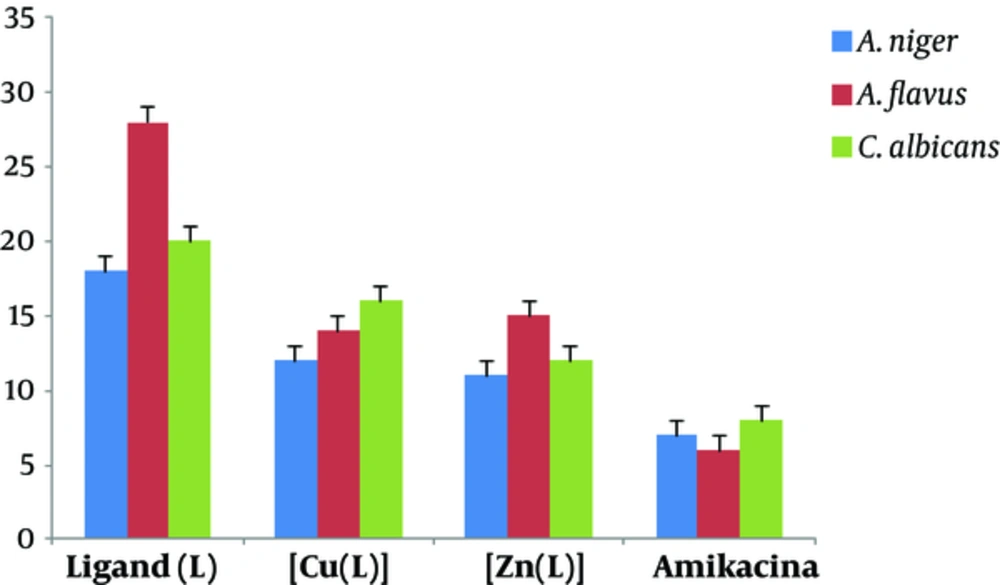
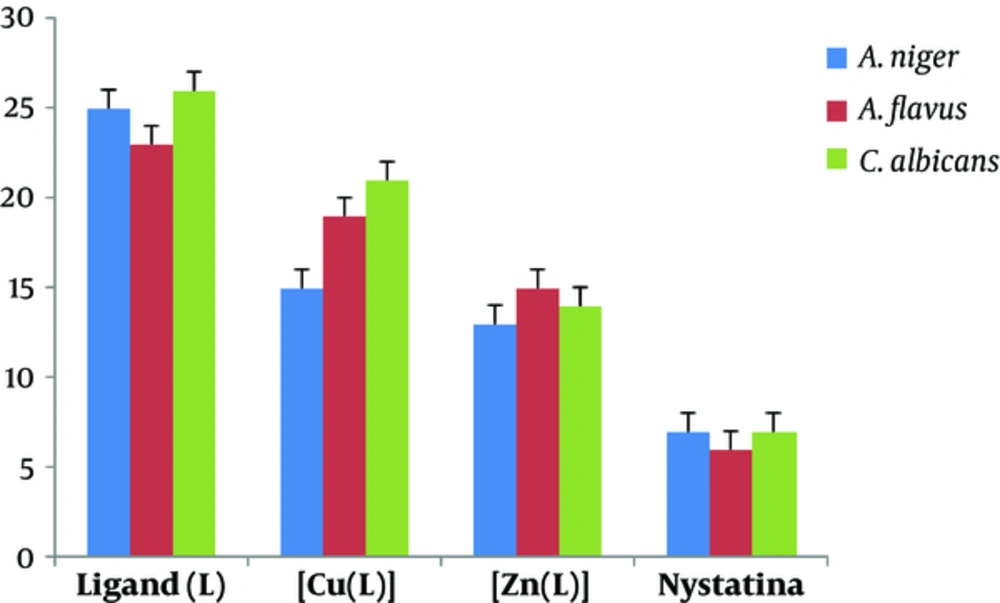
Synthesized ligand and its mononuclear Cu (II) and Zn (II) complexes were screened for in vitro antibacterial activities against Escherichia coli, Bacillus subtilis, and Staphylococcus aureus as well as antifungal activities against Aspergillus niger, Aspergillus flavus, and Candida albicans by the disc diffusion method. Amikacin and nystatin were used as the standard controls. Minimum inhibitory concentrations (MICs) were determined by the serial dilution technique (21). The nutrient broth bacterial and fungal cultures were incubated overnight at 37°C and room temperature, respectively, and growth rate was assessed in 24 and 48 hour intervals based on optical density (OD) (supplementary file appendix 6 and 7)
The antimicrobial studies of Schiff base metal complexes (Figures 7 and 8) showed that the complexes had higher activity than the ligand, which can be attributed to the complexation of the metal ions with the donor atoms of the ligand. In keeping with Tweedy conception (22, 23), chelation might improve the lipophilic nature of complex, which therefore favours its access through the lipid bilayers of the cell membrane and restricts the metal binding sites on the enzymes of microorganism. In vitro antibacterial studies demonstrated that copper (II) and zinc (II) complexes have superior biological (bacterial and fungal) activities in comparison with the ligand. Antimicrobial potentials can also be influenced via different ways beyond membrane permeability.
4. Conclusions
Analytical data revealed a tetrahedral geometry for the mononuclear complexes [Cu(C40H28N8O10)] and [Zn(C40H28N8O10)]. Metal complexes of Cu (II) and Zn (II) were synthesized from the ligand, analysed by spectral studies, and screened for their potential antimicrobial activity. Biological studies revealed that Schiff base complexes have enhanced biological activities than the free ligand. Chelation improved the lipophilic nature of the metal complexes and led it to pass through bacterial membrane (lipid membrane) more readily, and accordingly improved the inhibitory activity of the complexes.
![X-Ray Diffractogram of [Cu (C<sub>40</sub>H<sub>28</sub>N<sub>8</sub>O<sub>10</sub>)] X-Ray Diffractogram of [Cu (C<sub>40</sub>H<sub>28</sub>N<sub>8</sub>O<sub>10</sub>)]](https://services.brieflands.com/cdn/serve/3170b/8be020936825a62cea498460246d03af8f408df6/jjnpp-13-1-67179-i005-preview-preview.webp)
![X-Ray Diffractogram of [Zn(C<sub>40</sub>H<sub>28</sub>N<sub>8</sub>O<sub>10</sub>)] X-Ray Diffractogram of [Zn(C<sub>40</sub>H<sub>28</sub>N<sub>8</sub>O<sub>10</sub>)]](https://services.brieflands.com/cdn/serve/3170b/d77a33f67bbad2224211d2672cd5169f48dabd03/jjnpp-13-1-67179-i006-preview-preview.webp)