1. Background
Radiotherapy is one of the useful clinical procedures for the treatment of cancer patients. Approximately 70% of cancer patients receive radiotherapy in their treatment courses (1). External beam radiotherapy or brachytherapy constitutes a mainstay of therapy for patients with localized cancer. Although radiotherapy is a common choice for cancer therapy, various types of toxicities in irradiated normal tissues limit the received dose by tumor cells. The most sensitive organs against the toxic effects of radiation are the bone marrow, kidneys, gastrointestinal (GI) system, lungs, and others (2, 3). The detrimental effects of ionizing radiation on living systems occur mainly due to its indirect effects. After exposure to ionizing radiation, free radicals including Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) are generated by the radiolysis of water molecules. These free radicals can interact with various biomolecules immediately, leading to detrimental changes in their structure and function. The interaction of ROS and RNS with genomic content of cells can lead to death through apoptosis, mitotic catastrophe, and others. The massive cell deaths following exposure to radiation disrupt normal functions of irradiated organs (4).
The gastrointestinal tract is the second most vital organ after the hematopoietic system to the detrimental effects of radiation. As a result of a high dose of ionizing radiation, acute morphological changes to the intestine are observed within days to weeks (5). Early radiation enteropathy develops during radiation therapy as a result of intestinal crypt cell death, disruption of the epithelial barrier, and mucosal inflammation (6). The GI toxicity is the most important complication of radiotherapy of cancers within or adjacent to the pelvic area. Moreover, GI toxicity can cause threats to the lives of people who receive an acute radiation dose during a radiation accident like nuclear or radiological disasters. Exposing this organ to radiation can lead to some limiting side effects such as vomiting, bleeding, and diarrhea as a result of damage to stem cells, leading to epithelial atrophy and shortening of intestinal microvilli (5). However, there are no proved effective prophylactic or therapeutic agents to mitigate the symptoms of GI radiation injury.
Studies have proposed that inflammatory responses and chronic ROS production following exposure to radiation have central roles in intestinal damage. It has been shown that irradiating small intestinal cells can lead to chronic oxidative damage resulting from mitochondria dysregulation and NADPH oxidase upregulation (7, 8). The upregulation of inflammatory mediators such as cyclooxygenase-2 (COX-2) can amplify intestinal toxicity following irradiation (9). The inhibition of chronic inflammatory responses and redox activation has been proposed to reduce radiation toxicity in irradiated organs including the small intestine (10).
Many researchers have tried to mitigate radiation-induced intestinal toxicity using some radiation modifiers. However, the discovery and development of some radioprotectors of appropriate efficiency and low toxicity have been the aims of several studies in recent years. Some studies have shown promising results for some radioprotector agents such as melatonin, amifostine, curcumin, and some other agents with less toxicity (11-13). Metformin (1,1-dimethyl biguanide hydrochloride), a biguanide derivate, is the most widely prescribed drug for the treatment of hyperglycemia in individuals with type 2 diabetes. In recent years, some in vitro and in vivo studies have shown the ability of metformin to protect cells against ionizing radiation and to increase the therapeutic ratio of cancer radiotherapy (14-16). In addition, some studies have shown that metformin has some interesting properties such as antioxidant and anti-inflammatory properties (17, 18).
2. Objectives
In the current study, we aimed to evaluate the protective effects of metformin against radiation-induced small intestine injury.
3. Methods
3.1. Experimental Design
We purchased 20 Male Wistar albino rats weighing 200 ± 20 g from the Animal Laboratory of the Pharmacy School, Tehran University of Medical Sciences, Tehran, Iran. All animals were housed under constant temperature (21ºC) and photoperiod (12 h light/dark cycle). All animals had free access to standard rat chow diet. The rats were randomly allocated to four groups of five rats including A (control), B (radiation-treated only), C (metformin-treated only), and D (metformin-treated with radiation).
Metformin, purchased from Shimi Daru Company (Tehran, Iran), was dissolved in distilled water to give a dose of 20 mg per milliliter. Groups A and B received 1 ml serum physiologic while groups C and D were treated orally with metformin 24 hours before and 72 hours after irradiation (at a dose of 100 mg/kg body weight; single dose per day) using intragastric intubation.
3.2. Irradiation
Before irradiation, all rats were anesthetized by intraperitoneal injection of ketamine 90 mg/kg and xylazine 10 mg/kg. Irradiation was performed using a 60Co source at a Source-Skin Distance (SSD) of 80 cm while a single dose of 10 Gy was given at a depth of 1.5 cm (half thickness) with a dose rate of 101 cGy/min to the whole body in the supine position.
3.3. Histopathological Evaluation
All groups were decapitated 3.5 days after exposure to radiation. The duodenum and jejunum were removed and fixed in formalin 10%. For light microscopic studies, duodenum and jejunum specimens were embedded in paraffin and then, 5 µm sections were obtained and stained with Hematoxylin and Eosin (H & E). Atrophy and shortening of the villus, epithelial degeneration, and lifting were assessed and graded in a blinding manner by a histologist using the histologic injury scale previously defined by Hussein et al. (19).
3.4. Statistical Analysis
Data were analyzed by the ANOVA test and post hoc Tukey HSD using SPSS 16. The P values of < 0.05 were regarded as statistically significant.
4. Results
4.1. Effect of Metformin on Histopathological Damages in the Duodenum
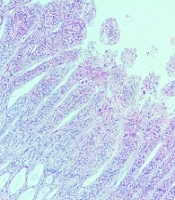
As shown in Figure 1, irradiating with 10 Gy gamma rays caused significant damage to the duodenum 3.5 days after exposure. Irradiation led to the significant shortening of villi and mucosal atrophy (P < 0.001), as well as edema and inflammation in villi (P < 0.001), without any significant epithelial degeneration (P = 0.40). Inflammation in the irradiated group spread to muscles. The results showed that exposure to radiation could not cause epithelial lifting. Moreover, the results did not show bleeding or vascular damage. The results related to treated rats with metformin before irradiation indicated that the shortening of villi and mucosal atrophy, as well as edema and inflammation, reduced significantly (P < 0.001). As the data show, the metformin-treated non-irradiated group had normal morphology. This implies that the treatment of rats with 100 mg/kg did not cause any cytotoxicity (Table 1).
| Groups | Villi Shortening, Mucosal Atrophy | Edema and Inflammation | Epithelial Degeneration |
|---|---|---|---|
| Control | - | - | - |
| Metformin | - | - | - |
| IR | + | + | Mild |
| Metformin + IR | Absent or mild | Absent or mild | - |
4.2. Effect of Metformin on Histopathological Damage in the Jejunum
As shown in Figure 2, irradiation with 10 Gy gamma rays caused significant damage to the jejunum 3.5 days after exposure. Exposure to this dose of ionizing radiation caused the significant shortening of villi and mucosal atrophy, edema and inflammation in villi, and epithelial degeneration (P < 0.01). The results showed that irradiation caused the spread of inflammation throughout the intestinal layers. However, the results indicated that irradiation did not lead to bleeding and vascular damage. Moreover, similar to the duodenum, the results showed that exposure to gamma rays did not exert any effect on epithelial lifting. The results related to treated rats with metformin before irradiation indicated that edema and inflammation in villi, as well as epithelial degeneration, reduced significantly (P = 0.03). However, it could not ameliorate villi shortening (P = 0.18). Similar to the duodenum, the metformin-treated group had normal morphology (Table 2).
| Groups | Villi Shortening, Mucosal Atrophy | Edema and Inflammation | Epithelium Degeneration |
|---|---|---|---|
| Control | - | - | - |
| Metformin | - | - | - |
| IR | + | + | + |
| Metformin + IR | Mild | - | - |
5. Discussion
Gastrointestinal toxicity is one of the most important side effects of cancer therapy. Moreover, it is the second cause of radiation-induced death after nuclear or radiological events. The jejunum is the most sensitive organ to gastrointestinal damage. However, duodenal damage has been observed among people who have received radiation for cancers adjacent to it. Small bowel toxicity after exposure to a high dose of radiation can cause acute or chronic radiation enteritis. Although acute enteritis may cause severe diarrhea and bleeding in a few days, chronic enteritis can lead to the appearance of some symptoms such as bloating, nausea, diarrhea, and bleeding long time after exposure. These side effects can strongly affect the quality of life of irradiated people. These side effects have been reported for half of patients that have undergone radiotherapy of upper-abdominal cancers (20).
The protection of the small bowel for preventing radiation toxicity is of utmost importance in radiobiology. So far, several different agents have been studied for reducing radiation toxicity in this organ. Moreover, in recent years, some studies have been conducted to mitigate radiation-induced death resulting from radiation-induced gastrointestinal syndrome. Some agents such as curcumin, melatonin, alpha-tocopherol, and simvastatin have shown radioprotective effects against radiation in small intestine injury (21-24). Moreover, studies in recent years have shown that supplementing with some antioxidants and radioprotectors after exposure to radiation can help mitigate gastrointestinal injury (25).
In the current study, we aimed to evaluate the possible radioprotective effects of metformin on the small intestine of rats. As expected, we observed that total body irradiation with a single dose of 10 Gy gamma rays led to extensive damages to both duodenum and jejunum. Treatment with metformin a day before irradiation for four consecutive days could ameliorate radiation toxicity in both duodenum and jejunum. We showed that treatment with metformin at 100 mg/kg for the mentioned period did not cause any cytotoxicity on the small intestine such as damage to villi structures. However, the administration of metformin could improve villous shortening, epithelial degeneration, and villi thickening. These results show that metformin can reduce damages to intestinal stem cells, edema, and inflammation.
Metformin has shown some protective properties against the detrimental effects of ionizing radiation. It has the ability to regulate cellular metabolism and reduce ROS production. In addition, metformin can scavenge free radicals directly or via the stimulation of antioxidant enzymes (26). Metformin has ability to stimulate DNA damage responses via AMP-activated protein kinase (AMPK). These properties are interested in the protection of normal tissues against radiation toxicity. In recent years, some studies have discovered the radioprotective effects of metformin in cells in vitro and in vivo. Cheki et al. showed that treatment of human T lymphocytes with metformin ameliorated apoptosis and DNA damage. They showed a 42% protection for 50 μM metformin concentration (27). Another in vivo study by Xu et al. showed that pre-treatment with metformin before irradiation suppressed chronic oxidative damage in mice bone marrow stem cells. The analysis showed that metformin blunts free radical production resulting from radiation-induced continuous upregulation of NADPH Oxidase 4 (NOX4). Moreover, they showed that metformin stimulated the activation of SOD, GSH, and catalase. Lastly, metformin could decrease stem cell senescence after radiation exposure (28). It seems that the inhibition of inflammation signaling pathways has a pivotal role in the protection of intestinal damage after exposure to radiation (29). For example, the inhibition of inflammasome can ameliorate enteritis and mucositis after irradiation (30, 31).
5.1. Conclusions
In this study, we showed that metformin can be proposed for the radioprotection of the small intestine. This property is very obvious, especially for damages to the villous and epithelium. However, further studies are needed for the protective mechanisms of metformin in this organ. It is possible that the inhibition of ROS production via mitochondria or inflammatory responses has a key role in the radioprotection of the small intestine by metformin.