1. Background
PD is a common disorder that causes damage to the central nervous system. It mainly affects the motor system and is generally diagnosed with the loss of dopaminergic neurons. In fact, the amount of dopamine in the striatum and hippocampus areas is decreased and leads to motor defects, including rigidity, tremor, and bradykinesia (1). Estrogen causes the striatal dopamine depletion in rats affected by MPTP and 6-OHDA. Although the research results have suggested the beneficial effects of estrogen in the treatment of PD, many women usually use phytoestrogens as an alternative to HRT (hormone replacement therapy) for avoiding the unwanted side effects (such as raised chance of endometrial and breast cancers and unstable bleeding) (2). Past studies have demonstrated the neuroprotective effects of traditional medicines against the cell and animal models of PD (3, 4).
Dopamine reacts with molecular oxygen to form dopamine-quinones, which can deplete the antioxidant glutathione. Producing reactive oxygen species (ROS) during this process as well as the enzymatic metabolic breakdown of dopamine (through monoamine oxidase) increases the formation of ROS (5). Whenever the production of ROS leads to the ability of the antioxidant system to remove them, the related alterations in both cognitive and motor skills will take place (6, 7). Therefore, many studies have been so far carried out to prove the role of the oxidative lesion in neurodegenerative diseases (such as PD) (8).
The brain damage induced by oxidative stress was found in both brain tissues from PD patients and in pharmacological models as well as reserpine models (9, 10). Many experimental evidences have already proved the role of oxidative stress as a mediator of nerve cell death in PD. Estrogens have been reported to facilitate the prevention of neuronal damages caused by increased oxidative burden. The major cause of PD is still unknown, however, post-mortem studies have firmly insisted on oxidative damage and mitochondrial impairment in the pathogenesis of PD (11). Recent studies have portrayed the role of estrogen for the protection in neurodegenerative diseases. Estrogen is associated with the reduced severity of symptoms in women with early-onset PD (12).
Moreover, clinical studies have stimulated the interests in antioxidant therapy based on the properties of estrogen or other similar estrogenic compounds (such as phytoestrogens). Di-phenolic compounds derived from plants are poorly bound to estrogen receptors (ER), which may exhibit the effect of estrogens or anti-estrogens. Besides that, it has antioxidant properties through hydrogen/electron donation via a hydroxyl group and may also act as free radical scavengers (phytoestrogens) (13). The effects of the combined gonadal hormone deprivation and cerebral hypoperfusion/ischemia on memory have been unrevealed. Hence, it was hypothesized that natural phytoestrogenic substance (e.g., Foeniculum vulgare) serves as a neurotrophomodulatory substance for some brain areas. In addition, neurons were thought to be included in the learning and memory processes. It should be known that gonadal hormones (like estrogen) can alter the cognitive performance (14), while ovarian hormones can differently effect the female memory in an age-dependent manner (15).
Surgically menopausal women and estrogen deficits in rats (ovariectomy) cause a cognitive damage, however the mechanism that causes such injuries has not been recognized yet (16). Estrogen deficit is associated with mental health disorders, emotional troubles, memory defects, and other cognitive failures (17). With regard to phytoestrogens, which have attracted a great deal of attention and interest, several lines of evidence have proved that they can present a protective effect against various diseases. This is one of the phytoestrogen compounds of the fennel plant, with a confirmed suppressive effect against many diseases. Some other studies, through conducting biochemical analyses, exhibited that the Foeniculum vulgare has antioxidant properties (18).
Foeniculum vulgare Mill (F. vulgare), regularly known as fennel, is a widespread medicinal plant from the family Apiaceae. The names used in traditional Iranian medicine for this plant are Razianeh, Razianaj, Badiyan, and Marsoun. The antioxidant activity of F. vulgare has been revealed by various studies (19, 20). Foeniculum vulgare exposes estrogen-like activities, following the oral administration of the acetone extract of F. vulgare fruit for 15 days in male rats (21). The essential oil of Foeniculum vulgare has demonstrated some beneficial effects in primary dysmenorrhea. There is some evidence in favor of the use of Foeniculum vulgare for the treatment of cognitive disorders, such as dementia and Alzheimer’s disease (22, 23).
Reserpine is an irreversible inhibitor of the vesicular monoamine transporter 2 (VMAT-2). The blockage of dopamine vesicular uptake results in the accumulation of neurotoxic dopamine oxidation byproducts (24). Reserpine injection to rats was suggested as a pharmacological model of PD, based on its effects on monoamine depletion and locomotor activity (25).
The present study investigated the effects of different doses of Foeniculum vulgare Mill on motor and behavioral disturbance in the model of PD, produced by reserpine in ovariectomized and non-ovariectomized rats.
2. Objectives
The fennel plant (Foeniculum vulgare Mill) belongs to the flowering plant of the Apiaceae family. It is a medicinal plant that is commonly used by the traditional tribes in Iran. The fennel plant has an estrogenic effect. The intention of this research is to analyze the effects of various doses of Foeniculum vulgare Mill on the behavioral and motor dysfunctions in the reserpine-induced model of PD in ovariectomized and non-ovariectomized rats.
3. Methods
3.1. Animals
Adult female Wistar rats, weighing approximately 250 - 300 grams, were used in this study. All of them were kept under the same conditions of temperature (22 ± 2 C) and humidity (55% - 60%) in the environment. The other conditions of 12 hours daylight and 12 hours darkness were also the same for everyone. Food pellets and water were available. All animal experiments were carried out in accordance with the NIH guide for the care and use of laboratory animals. The pharmacological protocols were approved by the institutional animal ethical committee (IAEC) of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1395.26), under the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA).
3.2. Drugs
Reserpine (Sigma-Aldrich, Germany) was initially prepared in acetic acid solution and was then diluted with distilled water. Levodopa and carbidopa (Sigma-Aldrich) were diluted in distilled water immediately before the injections. The essential oil of fennel was supplied from the company Barij, and the rats were orally administered.
3.3. Behavioral-Motor Tests
3.3.1. Open Field
The apparatus was a chamber with an open field area of 60 × 60 cm, with 25-cm high walls. It was made of wood and was painted black on the inside surface. We quantified the distance traveled (in meters), the frequency of rearing (partial or total rising onto the hind limbs), the immobility period (the duration their feet were completely moved from the initial state), the time in center (duration spent in the center of the open field), and grooming. On the 6th day, 24 hours after the 2nd injection of reserpine, the test was completed (26, 27).
3.4. Catalepsy Test
3.4.1. Bar Test
The catalepsy test was used for animals by putting the paws of both hands on the bar, which were 9 cm from the floor height. The period of catalepsy, which was defined as an immobile posture by keeping both forepaws on the bar, was measured up to a maximum of 180 seconds. For each animal, the experiment was repeated 3 times and each time, the observations were recorded. On the same day, the results were analyzed and the mean value of the 3 trials were recorded. The test, on the 6th day and 24 hours after the second injection of reserpine, was completed (26, 27).
3.5. Measuring the Amount of Esterogen in Non-Ovariectomized Rats and Ovariectomized Rats
On the 6th day of the study, after behavioral testing, the animals were anesthetized with ketamine and xylazine (sourced from the designated veterinarian), and the heart of each rat and blood samples (5 ml from each) were collected. The samples were centrifuged and the serums were subsequently separated. Afterward, the samples were frozen at -80 C and measured by the ELISA kit.
3.6. Procedure
3.6.1. Group of Animals
A total of 80 adult female rats were divided into 2 main groups, which included Non-ovariectomized groups (I, II, III, IV) and ovariectomized (V, VI, VII, VIII, IX and X). In Non-ovariectomized groups, the animal received reserpine (3 mg/kg) on the 3rd and 5th days, subcutaneously, except Group I, as Control group, just received normal saline orally for 5 days. Group II, III, and IV were also treated with oral saline¸ fennel essential oil (100 mg/kg), and L-dopa-carbidopa (20.5 mg/kg) for 5 consecutive days, respectively. On other hand, animals of the Ovariectomized groups (VI, VII, VIII, IX and X) received reserpine (3 mg/kg) on the 3rd and 5th days, subcutaneously. Then, these groups were also treated with oral saline, for 5 consecutive days and fennel essential oil (50 mg/kg), fennel essential oil (100 mg/kg), fennel essential oil (200 mg/kg), and L-dopa-carbidopa (20.5 mg/kg) for 5 consecutive days, respectively. Group V as the Control group received normal saline for 5 days orally.
3.7. Reserpine Effect in Ovariectomized Rats and Non-Ovariectomized
In order to observe the effects of reserpine on ovariectomized and non-ovariectomized rats, the rats were randomly allocated to 4 groups (N = 8). Two groups were treated with reserpine (3 mg/kg), whereas the other 2 groups did not receive any reserpine. The 4 groups are displayed here:
Group I: (NO-NS)
Group II: (NO-R)
Group V: (O-NS)
Group VI: (O-R)
Groups II (non-ovariectomized rats) and VI (ovariectomized rats) were subcutaneously administered with reserpine (3 mg/kg) on the 3rd and 5th days. Then, on the 6th day of the behavioral and motor activities, they were evaluated (26, 27).
3.8. Effects of Repeated Fennel Treatments on Reserpine-Induced Catalepsy
The groups were administered with the essential oils of fennel (50, 100, 200 mg/kg) for 5 consecutive days. On the 3rd and 5th days of the survey, they were subcutaneously administered with reserpine at a rate of 3 mg/kg. The best dose of the essential oil of fennel was used for ovariectomized rats, who displayed the best results. Afterward, on the 6th day, the animals were prepared for the bar test and the results were recorded. The following groups were involved in this part of the experiment:
Group III: (NO-R-F100)
Group VII: (O-R-F50)
Group VIII: (0-R-F100)
Group IX: (O-R-F200)
Female rats were surgically ovariectomized a month before the start of the study. By injecting ketamine (4 mg/kg) and xylazine (50 mg/kg), the rats were anesthetized and a half-centimeter cut was made in their abdomens. Then, using scissors, both ovaries were slowly removed from the fallopian tubes. Finally, the incision was sutured with surgical thread (chromic catgut 3 - 0) (28).
3.9. Parkinson’s Induced by Reserpine
In order to induce the symptoms of PD, the rats were subcutaneously with reserpine (3 mg/kg) in 2 stages, then, on the 3rd and 5th days, the motor symptoms of the animals were recorded. After 24 hours, the reserpine injection, the metabolism of dopamine was increased and thus, there was a low level of homovanillic acid and indole acetic acid (26, 27).
3.10. Statistical Analysis
This study aimed to investigate the difference between the measured quantities in the normal data by using one-way ANOVA statistical methods (P < 0.05) and in the non-normal data, the appropriate backup test was not normal.
4. Results
This research demonstrated the results of the intraperitoneal injection (IP) of reserpine and the effects on the locomotor activity in ovariectomized female rats and non-ovariectomized rats by using the related devices, open-field test, and bar test. Then, the recipient groups of the essential oils of fennel in different concentrations were compared.
4.1. Reserpine Injection Effect on Muscle Stiffness (Catalepsy)
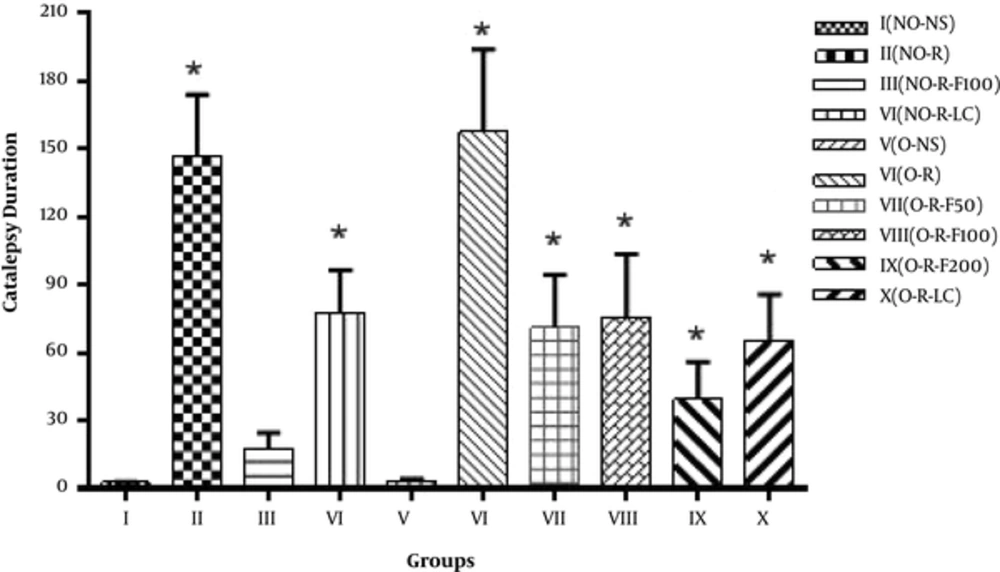
Table 1 portrays the muscle stiffness comparison between 10 groups through the bar test set-up, on the 6th day of the study, and 24 hours after the 2nd injection of reserpine. One-way ANOVA statistical analysis showed that there was a significant difference (P < 0.05) between Group I compared with other groups (II, IV, VII, VIII, IX and X). However, compared with Group V and group III, the difference was insignificant (P > 0.05). On the other hand, Group I presented a higher muscle stiffness compared to Groups V and III, consecutively. The statistical analysis expressed that this difference was not significant compared to the other groups (P > 0.05) (Figure 1).
| Groups | Esterogen Level | Bar Test | Openfild Test |
|---|---|---|---|
| I | 137.65 ± 10.73 | 2.63 ± 10.67 | 173.88 ± 5.15 |
| II | 129.97 ± 10.73 | 146.75 ± 10.67 | 4.25 ± 5.15 |
| III | 141.92 ± 10.73 | 17.67 ± 11.52 | 332.17 ± 5.56 |
| IV | 137.23 ± 10.73 | 77.75 ± 10.67 | 32.50 ± 5.51 |
| V | 107.32 ± 10.73 | 3.63 ± 10.67 | 166.87 ± 5.15 |
| VI | 106.32 ± 10.73 | 157.50 ± 10.67 | 3.88 ± 5.15 |
| VII | 120.35 ± 11.47 | 71.17 ± 11.52 | 27.50 ± 5.15 |
| VIII | 121.33 ± 11.47 | 75.50 ± 11.52 | 24.67 ± 5.56 |
| IX | 128.26 ± 10.73 | 39.67 ± 11.52 | 23.67 ± 5.56 |
| X | 110.15 ± 10.73 | 65.38 ± 10.67 | 16.38 ± 5.15 |
aValues are expressed as mean ± SEM.
4.2. Effects of Reserpine on the Locomotor Activity (Open Field Test)
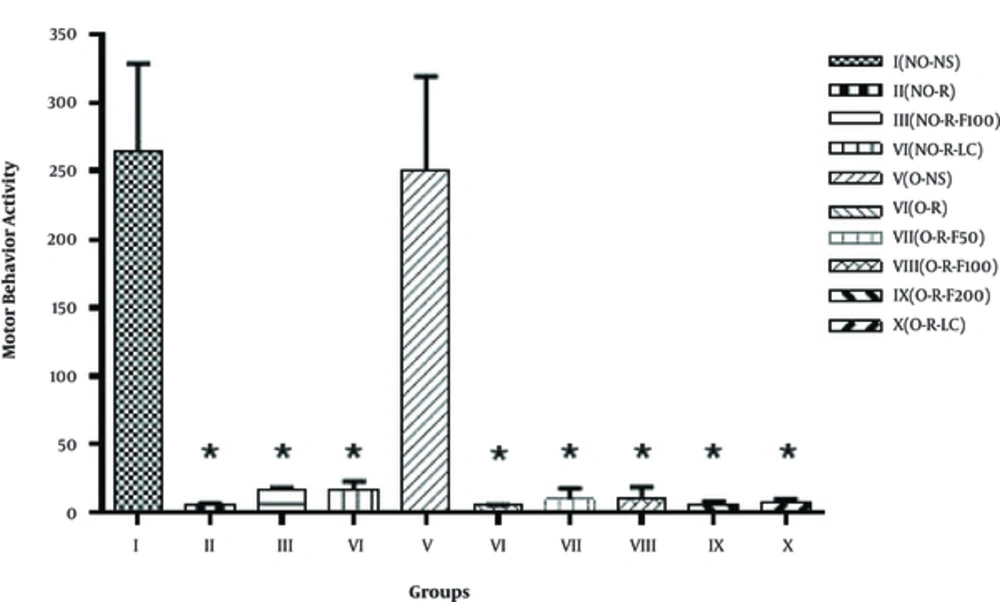
Table 1 reveals the comparison of the locomotor activity in open-field devices on the 6th day of the study and 24 hours after the 2nd injection of reserpine, between the 10 groups. One-way ANOVA statistical analysis showed that there was a significant difference (P < 0.05) between Group I and the other groups (II, III, IV, VI, VII, VIII, IX and X). However, compared with Group V, the difference was insignificant. Group I staged a better locomotor activity over group. The statistical analysis showed no significant difference between the other groups (Figure 2).
4.3. Ovariectomized and Fennel Effect on Estrogen Levels in Rats
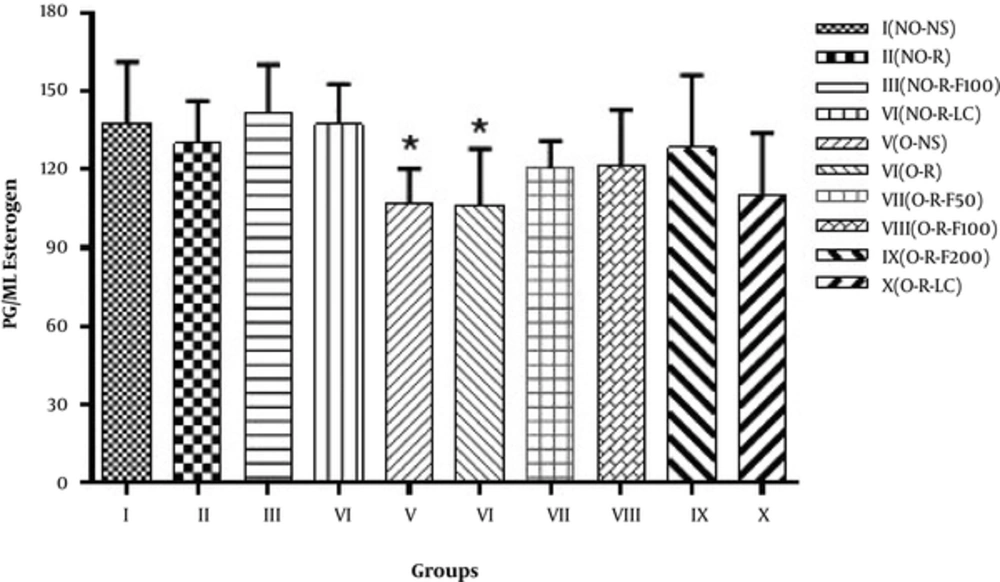
Figure 3 portrays the comparison of the estrogen level (pg/mL) in the studied groups of ovariectomized and non-ovariectomized rats, as well as the groups treated with the essential oil of fennel. One-way ANOVA statistical analysis disclosed that there was a statistically significant difference (P < 0.05) between Groups I and V and Group VI.
5. Discussion
In this study, the effect of reserpine injection (3 mg/kg) was evaluated in 2 phases on the locomotor and behavioral activities as a model of PD. In order to emphasize the importance of estrogen in the pathogenesis of the disease’s symptoms, the subsequent healing and therapeutic effects of the essential oil of fennel were tested and evaluated in this model, in ovariectomized female rats, and non-ovariectomized rats (Figures 1 - 3 and Table 1). The effect of reserpine injection on limb movement disorder can be more markedly observed in ovariectomized rats. Table 1 shows the disability of ovariectomized and non-ovariectomized rats and reveals the comparison between the groups that received reserpine. In fact, the locomotor and behavioral disorders 18 - 24 hours after each injection of reserpine is clearly visible.
The beneficial effects of the essential oil of fennel as an antioxidant and estrogenic product, which were investigated by Michael (1980) and Eun (2005), were also explored in this study. It was shown that the antioxidant effect of the essential oil of fennel can reduce the motor and behavioral disorders caused by the injection of reserpine. The reduction dose of 50 mg/kg and 100 mg/kg of the essential oil of fennel was evident compared to the other groups, which did not receive the essential oils. The essential oil of fennel was shown to have estrogenic effects (29).
Clinical studies on the role of estrogen in the pathogenesis of PD have expressed that sex hormones are principally involved. A variety of studies have demonstrated that estrogen inhibits the dopamine depletion in the brain striatal symptoms of Parkinson’s rats and is effective in eliminating the symptoms (1, 30, 31). In fact, estrogen-containing compounds have a protective role against neuronal injury (32). On the other hand, some other studies by Jeon et al. and Wang et al. have claimed that phytoestrogens prevent the degeneration of dopaminergic neurons and provide neuronal protection against the symptoms of Parkinson’s (33, 34). Estrogen has a protective effect on neurons and reduces the activity of microglial cells and inflammatory markers in the area of nigrostriatal injury and lowers the oxidative stress factors (34).
Various laboratory methods were used for detecting these types of studies. Similar to ovariectomized rats, a surgical menopause, which is a method for reducing the estrogen level in female rodents, can cause neurological complications and risks in animals (including oxidative damage and cognitive impairments) (35). Menopause is an age-related phenomenon that is associated with oxidative stress and free radical production. Failure to produce estrogen during this period can lead to neurodegenerative diseases (36). Estrogens are involved in the regulation of oxidative stress in the early stage of steroidogenesis. There is ample evidence that the mitochondria in the brain are the targeted steroids. In addition to its role in neurodegenerative diseases (such as Alzheimer’s and PD), sexual sensitivity and impaired mitochondrial function were unaffected (18).
Many studies have worked on the estrogenic properties of fennel (37). The gained results disclosed that ovariectomized rats significantly reduced the protective effects of estrogen in the neurodegenerative disturbance, induced by reserpine. In the present research, in ovariectomized rats (Group VI), which were administered with reserpine (3 mg/kg) on the 3rd and 5th days and also, received estrogen, the motor impairment was notable. It means that the amount of estrogen was low and the state of motion was in unsuitable conditions. In the Non-ovariectomized groups treated with fennel (Group 3) and Ovariectomized groups treated with fennel, in different doses of estrogen (Groups VII, VIII, and IX), the motor activity was in a better position. These results emphasize the role of estrogens and phytoestrogens in protecting dopaminergic neurons and improving the motor symptoms of PD (Figure 3 and Table 1). According to the current study, the protective effect of estrogen on dopaminergic neurons is evident.
In the previous researches with different laboratory models and cell cultures, it was shown that mitochondrial dysfunction is effectively involved in the pathogenesis of PD. It was also realized that the neurotransmitter dopamine is associated with the pathogenesis of this disease (38, 39). Several studies have exhibited that dopamine oxidation decreases the proteasome activity, ATP, and sleep, which causes the malfunction of the mitochondria (40, 41). According to the mechanism of the pathogenesis of PD, in connection with the mitochondrial damage and oxidative compounds, different roles (such as ROS in the pathogenesis of PD) have been reviewed in various animal models of Parkinson’s (such as the new models with reserpine).
It can be expected that the essential oil of fennel, through its antioxidant properties, can be effective in improving the symptoms. The reserpine increases in oxidative enzymes (e.g., MDA and GSH) in the brain are closely related to the pathogenesis of PD (42). In this study, it was comprehended that when reserpine was injected into the rats in different groups, the oxidative stress significantly increased, especially in ovariectomized rats. In the group, which was treated with the essential oils of fennel (50, 100, 200 mg/kg) and was non-ovariectomized (especially those who received 100 mg/kg of the essential oil of fennel and were administered with reserpine), Parkinson’s symptoms were observed. Many of these inhibitions of the oxidative stress displayed significant improvements in the motor and behavioral disorders.