1. Background
The aging society is one of the major social issues nowadays. Alzheimer’s disease (AD) and obesity are related factors that are recognized as the two main health problems (1, 2). It was reported that about 71% of Americans (people aged above 60 years) are overweight (3). Aging is a usual risk factor in the change of cholesterol homeostasis and prevalence of dyslipidemia. Furthermore, it is more significant in elderly people rather than young people (2). Various risk factors may affect biochemical features in aging and AD. In this respect, high fat diet (HFD) can exacerbate the severity of AD by affecting the vascular structure (4), increasing of oxidative stress, and alter the blood hemostasis (5). On the other hand, the formation of amyloid-β (Aβ) plaques in the blood vessels and brain parenchyma leads to some changes in the blood of the patients with AD and affect the blood hemostasis (6). Biochemical and hematological factors are expected to change in Alzheimer’s patients when they are taking HFD. These changes are probably more severe in Alzheimer’s patients who also take unhealthy food (i.e., HFD). Lower levels of RBC, hemoglobin (Hb), and hematocrit (HCT) have been observed in Alzheimer’s patients, as compared to the normal people (7). Abnormal hematological factors have also been observed in Alzheimer’s patients (7). On the other hand, HFD also affects the hematological factors (8). This type of diet significantly reduces the Hb concentration and the number of RBC in rabbit. However, the number of white blood cells (WBCs) and platelets significantly increases (8).
virgin coconut oil (VCO) is natural pure oil, containing useful compounds such as various types of flavonoids and polyphenols. It has been proven that VCO has strong anti-inflammatory, anti-oxidant, and anti-hyperlipidemic effects (9).
2. Objectives
In the present study, we evaluated the beneficial effects of VCO on the oxidative stress status, hematological, and some biochemical changes in Alzheimer’s and HFD rat models.
3. Methods
3.1. Animals
This study was performed by using 100 Wistar rats (250 ± 5 g, eight weeks old), which were purchased from Hamadan University of Medical Sciences (Hamadan, Iran). The animals were kept in animal’s house under standard conditions (21 - 25°C, and 50% - 60% relative humidity), with 12 hours under light/ 12 hours in dark. Rats had free access to water and food, and these experiments were conducted in accordance with the Ethics Committee of Kermanshah University of Medical Sciences (code: ir.kums.rec.1396.228).
3.2. Design of the Study
The rats were given a week to adapt to the environment. They were then randomly divided into the following 10 groups (n: 10/ group): group 1-healthy control; group 2-receiving Aβ (Alzheimer’s model); group 3-receiving Aβ + 8% VCO; group 4- receiving Aβ + 10% VCO; group 5-receiving HFD; group 6-receiving HFD + 8% VCO; group 7-receiving HFD + 10% VCO; group 8-receiving HFD + Aβ; group 9-receiving HFD + Aβ + 8% VCO; group 10-receiving HFD + Aβ + 10% VCO.
3.3. Diet Composition
The standard HFD (D12492, Research Diet) was bought from Royan laboratory animal feeds (Isfahan, Iran). The rodents’ standard dietary composition (chow diet) was used as a standard diet. According to the previous published papers, 8% and 10% VCO doses were used. These have been shown as effective doses and are the same usual VCO used by humans (2 - 3.5 tablespoons daily of VCO for a 72 kg man) (10, 11).
3.4. Preparation of VCO
VCO was prepared based on the method reported by Nevin KG et al. presented here with some modifications (12).
3.5. Surgery and Injection of Aβ
Aβ (1 - 40) (100 µg; Tocris Bioscience, Bristol, UK) was dissolved in 500 µL of normal saline (vehicle solution) and was kept at 37°C for seven days. This was done to induce peptide aggregation and fibril formation (13). Rats, which had been transferred to a stereotaxic apparatus (Stoelting Co., Wood Dale, IL, USA), were anesthetized with intra peritoneal injection of ketamine (100 mg/kg) and Xylazine (10 mg/kg). During the surgery, the body temperature of the rats was maintained at 37.0 ± 0.2ºC. However, based on the Paxinos Atlas (0.8 mm posterior to bregma; 1.5 mm lateral to sagittal suture; 3.6 mm beneath the surface of brain), the left ventricle was located. A volume of 5 µL was injected slowly into the left ventricle in 5 minutes.
3.6. Sample Collection
Eight weeks after HFD and VCO administration, blood samples were collected from the heart of the rats, followed by anesthesia with diethyl ether. Blood samples were collected in the tube containing 3.2% sodium citrate and immediately used for measuring hematological factors. Other tubes without anticoagulant were used for serum preparation and biochemical factors.
3.7. Total Antioxidant Capacity (TAC)
TAC in the serum was detected by measuring ferric reducing antioxidant power (FRAP) assay. In this method, antioxidant compositions of the sample reacted with the ferrous-tripyridyltriazine (TPTZ), which was composed of Fe+3-TPTZ. The blue color of the reaction revealed the TAC value in the samples. Reduction of ferric ions (Fe3+) and their conversion to ferrous (Fe2+) were assessed by spectrophotometry at 593 nm.
3.8. Total Oxidant Status (TOS)
The oxidants present in the samples oxidized the ferrous ion (Fe2+) into the ferric ion (Fe3+). The reaction of ferric ion with xylenol orange formed a colorful complex in an acidic solution. The measurement of light absorption of the colorful mixture via spectrophotometer could be used for evaluating the aggregate totality of oxidant molecules, which are present in the samples.
3.9. Measurement of Hematological Factors
Complete blood count (CBC) was performed through a fully automated cell counter device (SE Sysmex, KX21N). Differential count was performed after blood smear preparation and after Wright and Giemsa staining.
3.10. Measurement of the Amount of Serum Calcium (Ca) and Phosphate
The serum Ca and phosphate concentration were measured by using commercial diagnostic kit, according to the manufacturer’s instruction (Pars Azmoon Co., Iran).
3.11. Statistical Analysis
Statistical analysis was performed by using Statistical Package for Social Science software (SPSS version 16.0, SPSS). Results were expressed as mean ± SD, with significance defined as P < 0.05. The data were analyzed using two-way analysis of variance (ANOVA), Tukey multiple comparison tests were used to analyze the significance of the differences between the groups.
4. Results
4.1. Effect Aβ and HFD on TAC and TOS
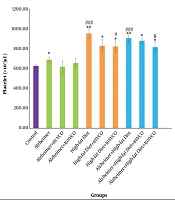
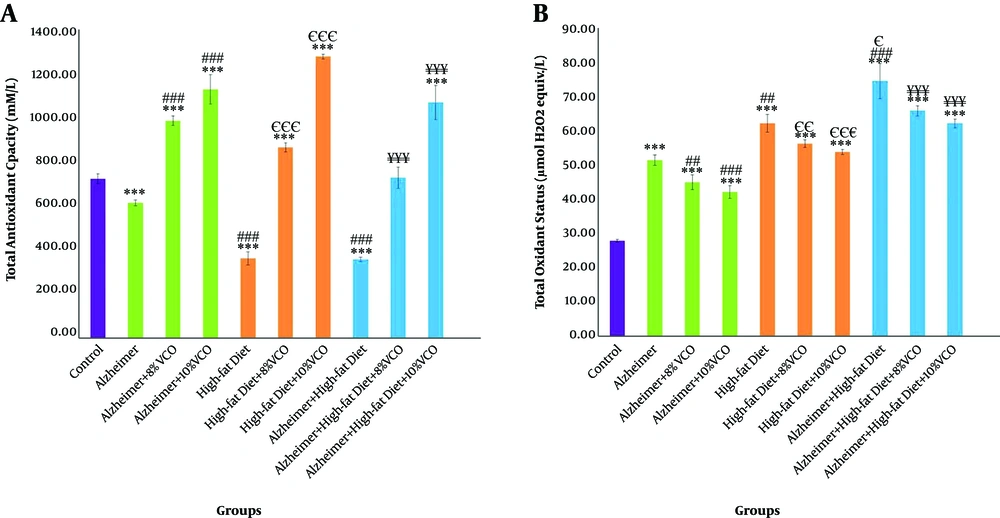
TAC level significantly reduced in Aβ and HFD treated-animals as compared to the control group (P < 0.001), and treatment with 8% and 10% VCO increased TAC in these groups (P < 0.001). Difference between 8% and 10% VCO is significant (P < 0.01), and this enhancement was more obvious by using 10% VCO (Figure 1A). As shown in Figure 1B, TOS level was increased in the Alzheimer group, HFD group, and HFD + Alzheimer group in comparison with the control group (P < 0.001). In the HFD group (P < 0.01) and HFD + Alzheimer group (P < 0.001), this factor was significantly increased, compared to the Alzheimer group. Treatment with 8% and 10% VCO significantly reduced the TOS levels (P < 0.001).
Change of serum’s total antioxidant capacity (TAC) (A) and total oxidant status (TOS) (B) in the studied groups. Results are presented as the mean ± SD of mean (P < 0.05). *** P < 0.001, ** P < 0.01 and * P < 0.05 compared with control group. ### P < 0.001, ## P < 0. 01 and # P < 0.05 compared with Alzheimer group. €€€ P < 0.001, €€ P < 0.01 and € P < 0.05 compared with HFD group, and ¥¥¥ P < 0.001, ¥¥ P < 0.01 and ¥ P < 0.05 compared with HFD+ Alzheimer group.
4.2. Effect of Aβ and HFD on White Blood Cells (WBC) and Lymphocyte (Lym) Count
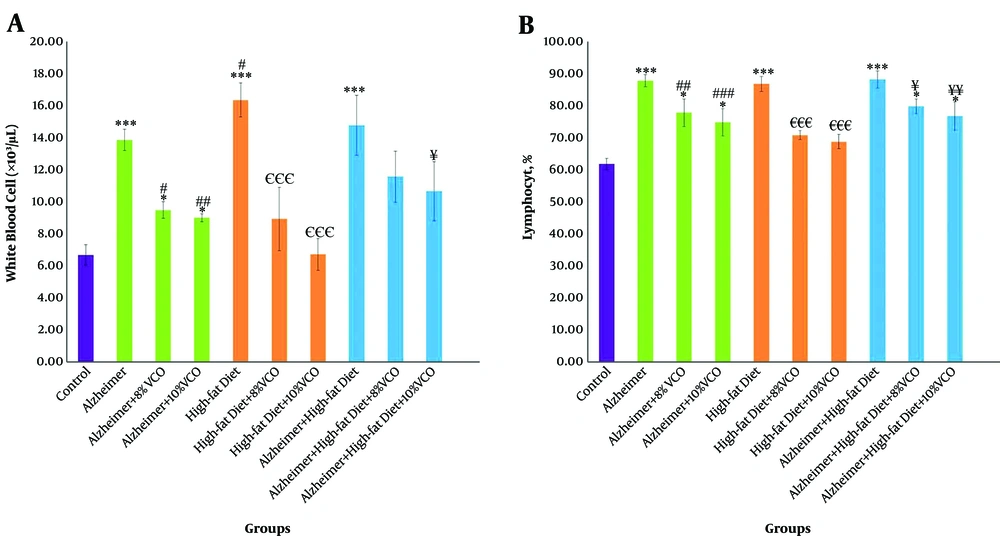
WBC and Lym were significantly increased in the Alzheimer group, HFD group, and HFD + Alzheimer group in comparison with the control group (P < 0.001), (Figures 2A and B). However, the high-fat diet increased WBC, more than amyloid-β (P < 0.05). Feeding of rats with 8% VCO and 10% VCO significantly decreased the WBC and Lym count in these groups. In addition, using a dose of 10% VCO was more effective for these reductions.
Changes of white blood cells (WBC) count (A) and lymphocyte (Lym) percentage (B) in the studied groups. Results are presented as the mean ± SD of mean (P < 0.05). *** P < 0.001, ** P < 0.01 and * P < 0.05 compared with control group. ### P < 0.001, ## P < 0.01 and # P < 0.05 compared with Alzheimer group. €€€ P < 0.001, €€ P < 0.01 and € P < 0.05 compared with HFD group, and ¥¥¥ P < 0.001, ¥¥ P < 0.01 and ¥ P < 0.05 compared with HFD+ Alzheimer group.
4.3. Effect of Aβ and HFD on Red Blood Cells (RBC), Hb, and HCT
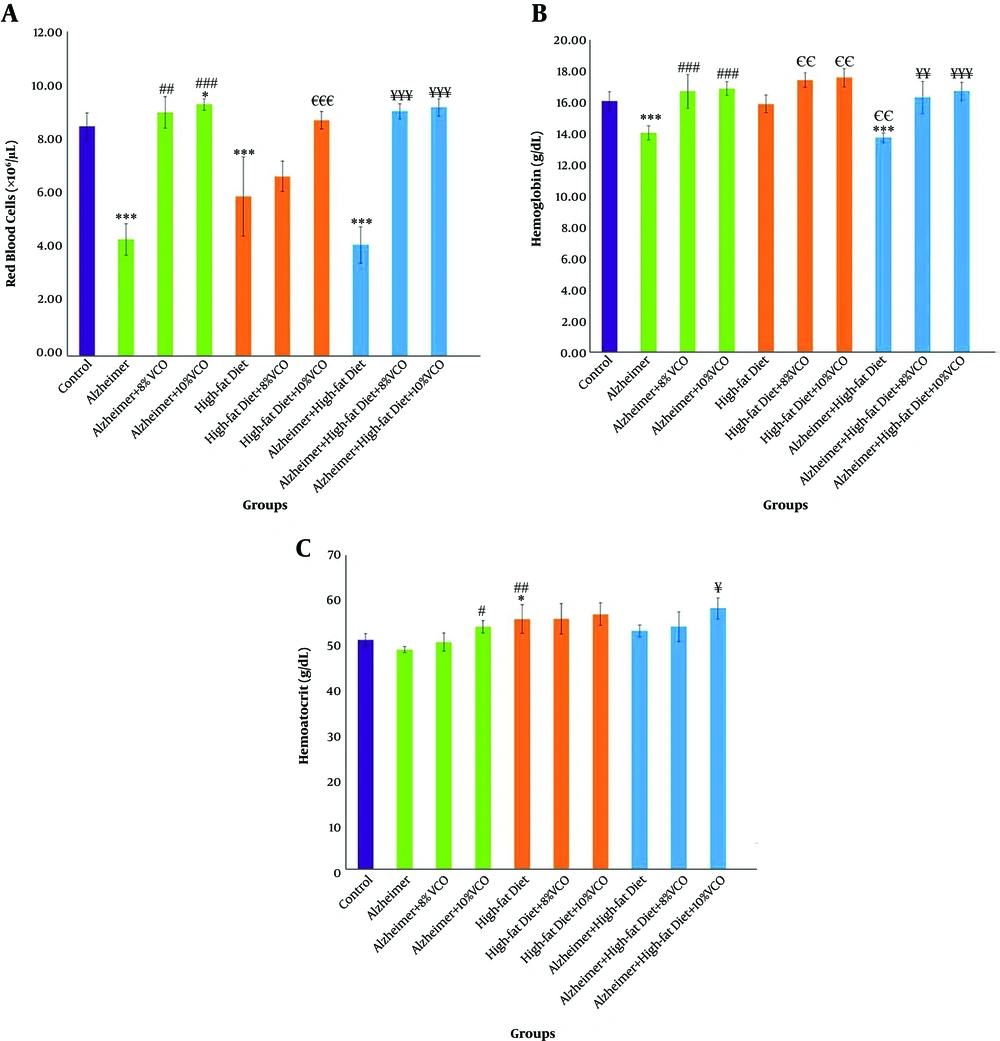
Figure 3A shows that the RBC count was significantly decreased in the Alzheimer, HFD, and HFD + Alzheimer groups in comparison with the control group (P < 0.001) and the difference between the three groups was not significant. Treatment with 8% and 10% VCO significantly normalized RBC count in these groups (P < 0.01 and P < 0.001). Moreover, Hb was significantly decreased in the Alzheimer group and Alzheimer+ HFD group (P < 0.001) (Figure 3B), compared to the control group. A total of 8% and 10% VCO significantly increased Hb all groups (P < 0.01).
Changes of red blood cell (RBC) count (A), hemoglobin (Hb) (B) and hematocrit (HCT) (C) in the studied groups. Results are presented as the mean ± SD of mean (P < 0.05). *** P < 0.001, ** P < 0.01 and * P < 0.05 compared with control group. ### P < 0.001, ## P < 0. 01 and # P < 0.05 compared with Alzheimer group. €€€ P < 0.001, €€ P < 0.01 and € P < 0.05 compared with HFD group, and ¥¥¥ P < 0.001, ¥¥ P < 0.01 and ¥ P < 0.05 compared with HFD+ Alzheimer group.
As depicted in Figure 3D, no significant change of HCT was shown in the Alzheimer group, whereas it significantly increased in the HFD group (P < 0.05). Furthermore, 10% VCO significantly increased HCT in the Alzheimer and Alzheimer+ HFD groups (P < 0.05).
4.4. Effect of HFD and Aβ on Changes of MCV, Mean MCH, and MCHC
The MCV, MCH, and MCHC in the Alzheimer group, HFD, and HFD + Alzheimer groups decreased, but these changes were not significant in comparison with the control group (P > 0.05), (Table 1). Moreover, treatment with 8% VCO and 10% VCO normalized these factors.
| Group | MCV, Fl | MCH, Pg | MCHC, g/dL |
|---|---|---|---|
| Control | 59.77 ± 1.83 | 18.65 ± 0.53 | 31.92 ± 0.24 |
| Alzheimer | 56.17 ± 0.77 | 18.12 ± 0.36 | 29.82 ± 0.69 |
| Alzheimer + 8% VCO | 57.23 ± 1.25 | 18.56 ± 1.07 | 30.25 ± 0.48 |
| Alzheimer + 10% VCO | 59.32 ± 0.67 | 18.7 ± 2.28 | 30.97 ± 1.790 |
| High-fat diet | 57.19 ± 3.24 | 18.34 ± 0.28 | 31.79 ± 1.00 |
| High-fat diet + 8% VCO | 58.62 ± 2.02 | 18.62 ± 1.86 | 32.01 ± 1.07 |
| High-fat diet + 10% VCO | 59.6 ± 0.27 | 18.73 ± 1.52 | 32.91 ± 1.62 |
| Alzheimer + high-fat diet | 57.12 ± 1.39 | 18.17 ± 0.50 | 30.07 ± 0.09 |
| Alzheimer + high-fat diet +8% VCO | 57.93 ± 0.41 | 18.24 ± 0.17 | 30.85 ± 0.84 |
| Alzheimer + high-fat diet +10% VCO | 58.64 ± 1.29 | 18.2 ± 1.13 | 31.47 ± 1.40 |
Abbreviations: MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; VCO, virgin coconut oil.
aResults are presented as the mean ± SD of mean (P > 0.05).
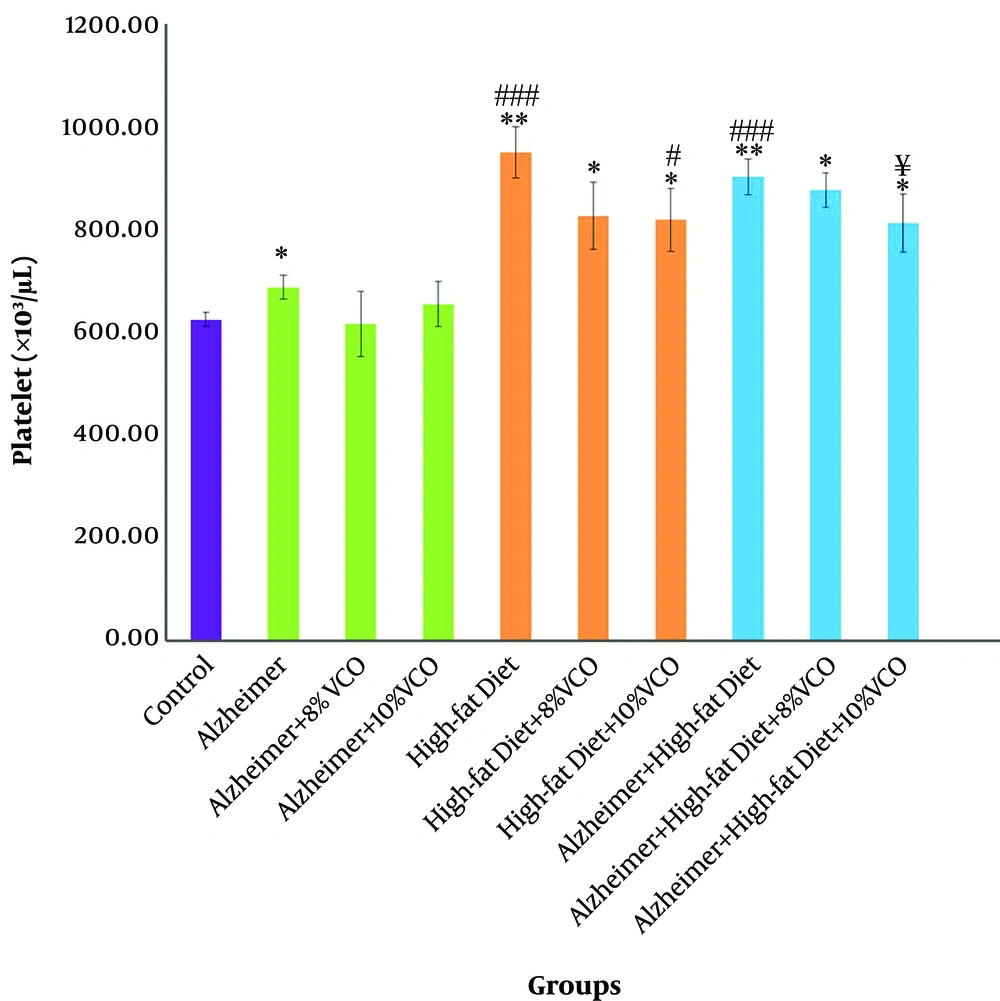
4.5. Effect of Aβ and HFD on Platelet Count Change
Aβ and HFD significantly increased platelet count in comparison with control group (P < 0.05). The administration of 10% VCO (P < 0.001) caused a significant reduction in platelet count as compared to the HFD and HFD + Alzheimer group (P < 0.05) (Figure 4).
Change of platelet count in the studied groups. Results are presented as the mean ± SD of mean (P < 0.05). *** P < 0.001, ** P < 0.01 and *P < 0.05 compared with control group. ### P < 0.001, ## P < 0.01 and # P < 0.05 compared with Alzheimer group. €€€ P < 0.001, €€ P < 0.01 and € P < 0.05 compared with HFD group, and ¥¥¥ P < 0.001, ¥¥ P < 0.01 and ¥ P < 0.05 compared with HFD+ Alzheimer group.
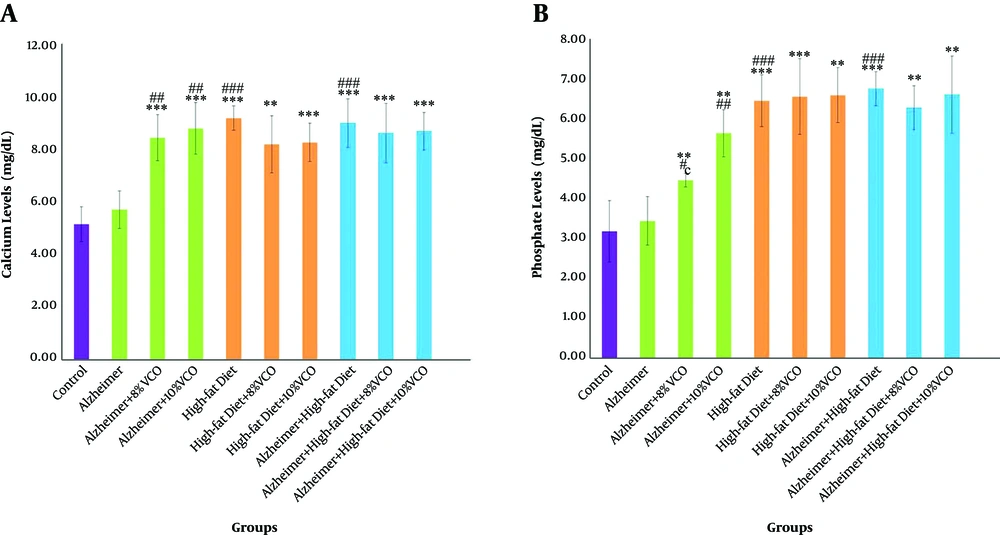
4.6. Effect of HFD and Aβ on the Changing of Ca and Phosphate Levels
The effect of Aβ on serum Ca and phosphate concentration was insignificant (P > 0.05). However, a substantial enhancement in serum Ca and phosphate concentration was observed in the HFD and HFD + Alzheimer groups as compared to the control group (P < 0.001). Additionally, 8% and 10% VCO diet caused a considerable increase in these factors in comparison with the control group and Alzheimer group (Figure 5A P < 0.01 and Figure 5B P < 0.001).
Changes of Calcium (Ca)(A), Phosphate (B) levels in the studied groups. Results are presented as the mean ± SD of mean (P < 0.05). *** P < 0.001, ** P < 0.01 and *P < 0.05 compared with control group. ### P < 0.001, ## P < 0. 01 and # P < 0.05 compared with Alzheimer group. €€€ P < 0.001, €€ P < 0.01 and € P < 0.05 compared with HFD group, and ¥¥¥ P < 0.001, ¥¥ P < 0.01 and ¥ P < 0.05 compared with HFD+ Alzheimer group.
5. Discussion
Aβ and HFD induced oxidative stress condition in serum, and VCO improved these effects. The antioxidant effect of VCO can be attributed to the compounds, which are present in this oil, such as flavonoids and polyphenols (14). Oxidative stress causes direct or indirect reactive oxygen species (ROS)-mediated damage of nucleic acids, proteins, and lipids, and ultimately causes cell death (15).
In this study, both Aβ and HFD decreased the number of RBCs, and HFD exacerbated the effect of Aβ. It has been shown that HFD increased Met-hemoglobin and, consequently, auto-oxygenize significantly increased this. In turn, it changed the cell membrane lipids and increased the deletion of these cells from blood circulation. In fact, the fragility of the RBC membrane, following structural changes in the membrane, removed a large number of RBCs in the spleen and ultimately reduced the number of RBCs accompanying HFD consumption. The composition of VCO shows strong antioxidant properties. Moreover, VCO has positive effects on lipid profiles and, probably in this way, indices on RBC health (12).
According to the findings of this study, the increase of WBC and Lym count in groups that had received Aβ and HFD indicates an inflammation condition in the serum. Various studies have concluded that the use of an HFD causes overweight, obesity, increases leptin concentration (16), and ultimately induces chronic inflammation, which is associated with an increase in the WBC count (17). An increase in the number of WBC and Lym in the Alzheimer group can also be attributed to an increase in inflammatory conditions following the increase of ROS in the serum. In this study, VCO significantly restored WBC and Lym count. This decrease is most likely due to the anti-inflammatory effect of this oil (9).
As mentioned above, in the groups that had been received Aβ and HFD, Hb decreased. HFD through increased the ROS, enhanced the auto-oxidation rate of Hb, and finally promoted the conversion of HbO2 hemoglobin and the fractions of unstable Hb molecules to Met-Hb and carboxyhemoglobin (8). In this study, it showed that Hb levels and cell volume were lower in Alzheimer group compared to the normal rats. Hb has been seen with Aβin amyloid plaques in the brain of Alzheimer patients (18, 19). In addition, Aβ binds to Hb through the iron-containing parts (20). In the brain of the elderly and Alzheimer patients, following blood-brain barrier damage, the Hb migrates from the peripheral blood stream to the brain (21), and probably Aβ and HFD in this mechanism are able to reduce the Hb level. Our results indicated an enhancement of platelet count in the HFD group, Alzheimer group as well as the HFD + Alzheimer group. This suggests that platelet apoptosis, caused by the increase of Aβ, also alters blood homeostasis, removes platelets from the blood circulation, and is absorbed by phagocytosis cells (22). As our results contradict this, it would be concluded that for observation of the effects of Aβ on the platelet, more than 8-weeks time or more concentration of Aβ is needed. VCO normalized these changes by regulating lipid profiles and oxidative status and decreasing the inflammation. Since Ca concentration significantly changed in Alzheimer’s and dyslipidemic people, normalizing of this ion may involve the complications of these disorders. This study’s results show that consumption of HFD increased the concentration of Ca and phosphate in the serum. In fact, the level of Ca in the serum increases with the activation of the osteoclasts. Therefore, it is likely that HFD increases the activity of the osteoclasts, and serum Ca levels would be increased (23). Alzheimer’s patients have been found to have a lower level of Ca compared to normal individuals at the same age (24); however, in this study, Aβ injection did not alter the level of Ca and phosphate in the serum. This lack of change could be justified in several ways. It is possible that during the 8-week period, the changes triggered by Aβ and amyloid plaques had not been sufficient, or the dose of injectable Aβ did not affect the serum level of Ca and phosphate. Our results also indicated that VCO increased the serum Ca and phosphate concentrations in the Alzheimer group. Some studies have shown that the intestinal absorption of Ca appears dependent on the amount and type of saturated fatty acids (25, 26). Since this oil has been proven to increase Ca concentration in the bones, it can be concluded that VCO probably increases Ca uptake from the intestine.
5.1. Conclusions
Amyloid-β and the consumption of high-fat diet significantly changed blood cells and increased reactive oxygen species. Virgin coconut oil can normalize these changes.