1. Background
The blood-brain barrier (BBB) plays a protective role for the brain by limiting the entry of neurotoxic blood-derived products into the brain (1). This layer that consists of specialized endothelial cells and surrounding pericytes and astrocytes is so sensitive to inflammatory insults, which can result in numerous neurocognitive syndromes (2). Several factors can destroy the BBB of which inflammation, that appears immediately following the insult, is the most common cause (3). Inflammation in the brain is one of the most causative factors in neurological diseases like depression (4), Alzheimer (5), multiple sclerosis (6) and Parkinson (7). It also seems to be responsible for behavioral responses that are known collectively as sickness behavior such as impairment of cognitive abilities, fatigue, and malaise, decreased locomotor, body weight and appetite (8). Inflammatory markers, including interleukin (IL)-1β, tumor necrosis factor (TNF-α), and IL-6, which communicate with the central nervous system (CNS) by vagal afferents and crossing the BBB, damage synapses and neurons, and ultimately lead to cognitive dysfunction (9). Lipopolysaccharide (LPS), is a piece of Gram-negative bacteria and is well-known as a potent inducer of inflammation. It is suggested to be able to increase BBB permeability by releasing cytokines (e.g., TNF-α, IL-6, IL-1β), prostaglandin E2, and nitric oxide (NO) (10, 11).
Anti-inflammatory agents are suggested to be useful to protect the BBB. Thymoquinone (TQ) a well-known component of Nigella sativa (NS) has been frequently reported to have anti-inflammatory effects (12-14). Other therapeutic effects of TQ consist of neuroprotective, antioxidant, anticonvulsant, and anti-tumor activity (15, 16).
2. Objectives
In this study, protection against BBB permeability, as a possible mechanism for protective effects of TQ against sickness behaviors induced by LPS, was investigated in rats.
3. Methods
3.1. Animals and Treatments
Male Wistar rats were obtained from a local animal center at Mashhad University of Medical Sciences for the experiments. They had a 70 - 80 days old and 240 ± 10 g weight. They were housed under standard status (temperature 22 ± 2°C, humidity of 54 ± 2%, and 12 h light/dark cycle). Food and water were freely accessible. Fifty of the rats were divided into five groups and treated (n = 10 in each group): (1) The animals in the control group received saline; (2) the rats in LPS group were injected by LPS (1mg/kg/day; i.p.); (3-5) the animals in these three groups received different doses of TQ (2 mg/kg in LPS-TQ 2, 5 mg/kg in LPS-TQ 5, and 10 mg/kg in LPS-TQ 10 group) and were then injected by LPS. The treatments were done for one week and were continued during behavioral tests. TQ was administered 30 min before LPS. LPS was injected 120 min before Behavioral tests. Both LPS and TQ were obtained from Sigma-Aldrich Company (Sigma Chemical Co). The drugs were dissolved in sterile saline prior to injection.
3.2. Behavioral Procedures
One week after the injection, an activity of the animals was evaluated in open-field (OF) by placing the rats into the center of a clear Plexiglas (100 × 100cm) for 5 min. A dim light was provided in a room and open-field was done. Activity was quantitated by a digital camera and the parameters such as the number of crossing in the central and peripheral zones, the traveled distance in central and peripheral zones, the time spent in central and peripheral zones, the total crossing number and traveled distance were recorded (16).
Elevated plus-maze (EPM) was a device designed with two closed and two open arms. The device was set 100 cm above the floor. Each rat was placed in the center of the arms and allowed to move inside the arms. Finally, the time spent and the number of entries into the open and closed arms were recorded. Forced Swimming test (FST) was performed for all groups as previously reported (16). In summary, each rat was compelled to swim in a water-filled tank. The total duration of immobility, as well as active and climbing times were recorded.
3.3. Blood-Brain Barrier Permeability
After completing the tests, the animals were anesthetized with urethane (1.6 g/kg) (17), then 20 mg/kg Evans blue dye was injected intravenously. After circulating for 20 minutes, in order to separate the brain, they were slaughtered. After launder with saline and drying, the tissues (cortex, cerebellum, and brain stem) were separately weighed and immersed in 5 mL formamide for 72 hours (18). The samples were homogenized with 2 ml trichloroacetic acid (TCA) 50% and then centrifuged (1500 rpm, 10 min). The absorbance of each tissue was measured at 632 nm (19).
3.4. Statistical Analysis
The data were presented as mean ± standard error of the mean (SEM). One-way ANOVA and Tuckey post hoc comparison tests were carried out. The P < 0.05 was considered statistically significant.
4. Results
4.1. Open-Field
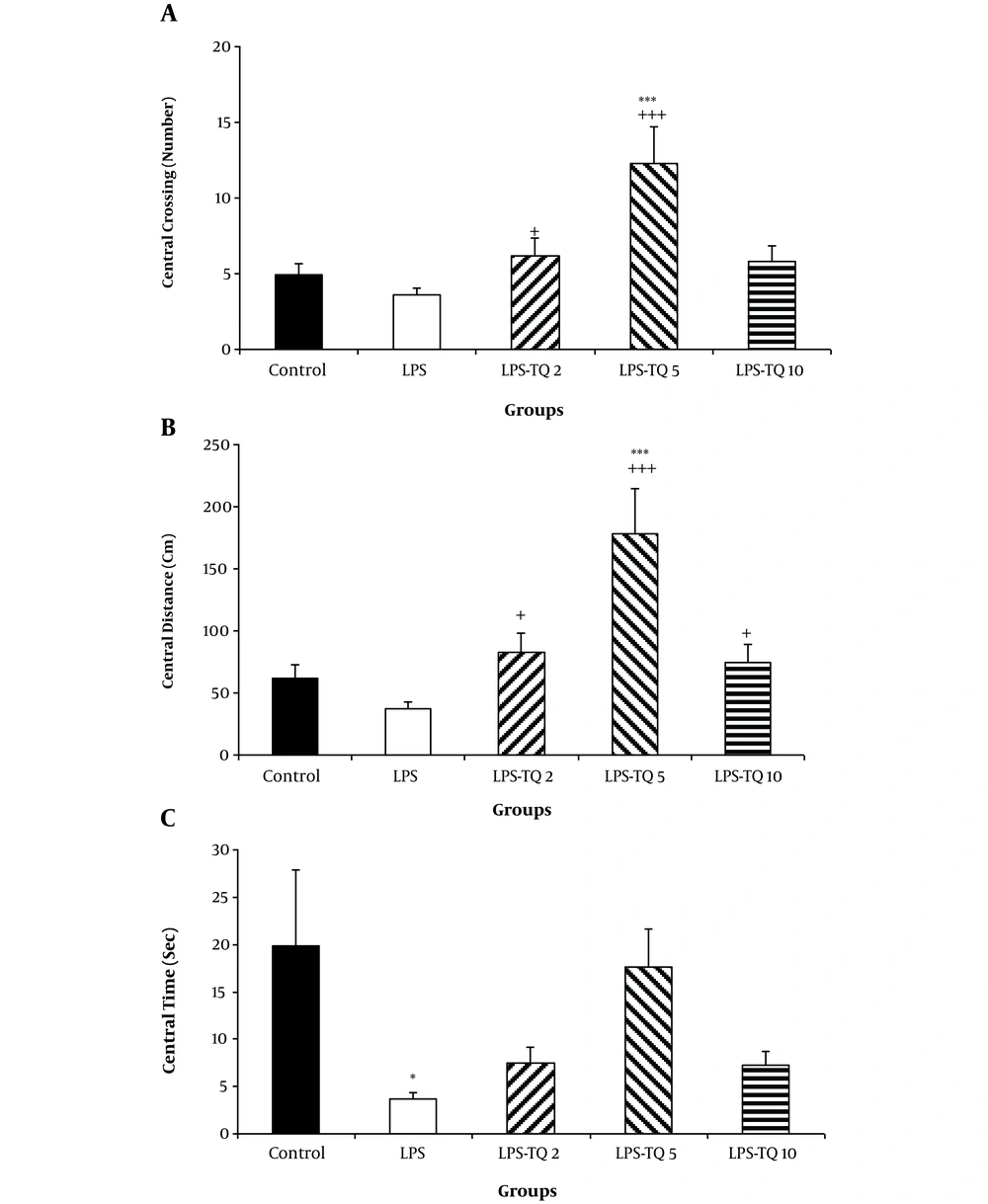
According to the results, no significant difference was observed in the central crossing number between LPS and control groups (Figure 1A). The crossing number in the central zone by the animals of LPS-TQ 2 and LPS-TQ 5 groups was significantly more than the LPS group (P < 0.05-P < 0.001; Figure 1A); however, no significant difference was observed between LPS-TQ 10 and LPS groups (Figure 1A). The results also showed that the animals of LPS-TQ 5 group had a higher crossing number compared to the control group (P < 0.001). Central zone traveled distance was not significant between the LPS and control groups; however, this parameter in all three TQ-treated groups was more than LPS group (P < 0.05 - P < 0.001; Figure 1B). The animals of LPS-TQ 5 group had a longer central distance compared to the control group (P < 0.001). As shown in Figure 1C, the animals of the LPS group also spent a significant (P < 0.05) lower time in the central segment than the control group. However, treatment with TQ reversed this LPS-induced change and there was no significant difference between the LPS-TQ (2, 5, 10) and LPS groups.
A, Crossing; B, Distance; and C, Time in the central zone in the open field test. Data are shown as the mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; ***, P < 0.001 compared to the control group; +, P < 0.05; and +++, P < 0.001 compared to the LPS group.
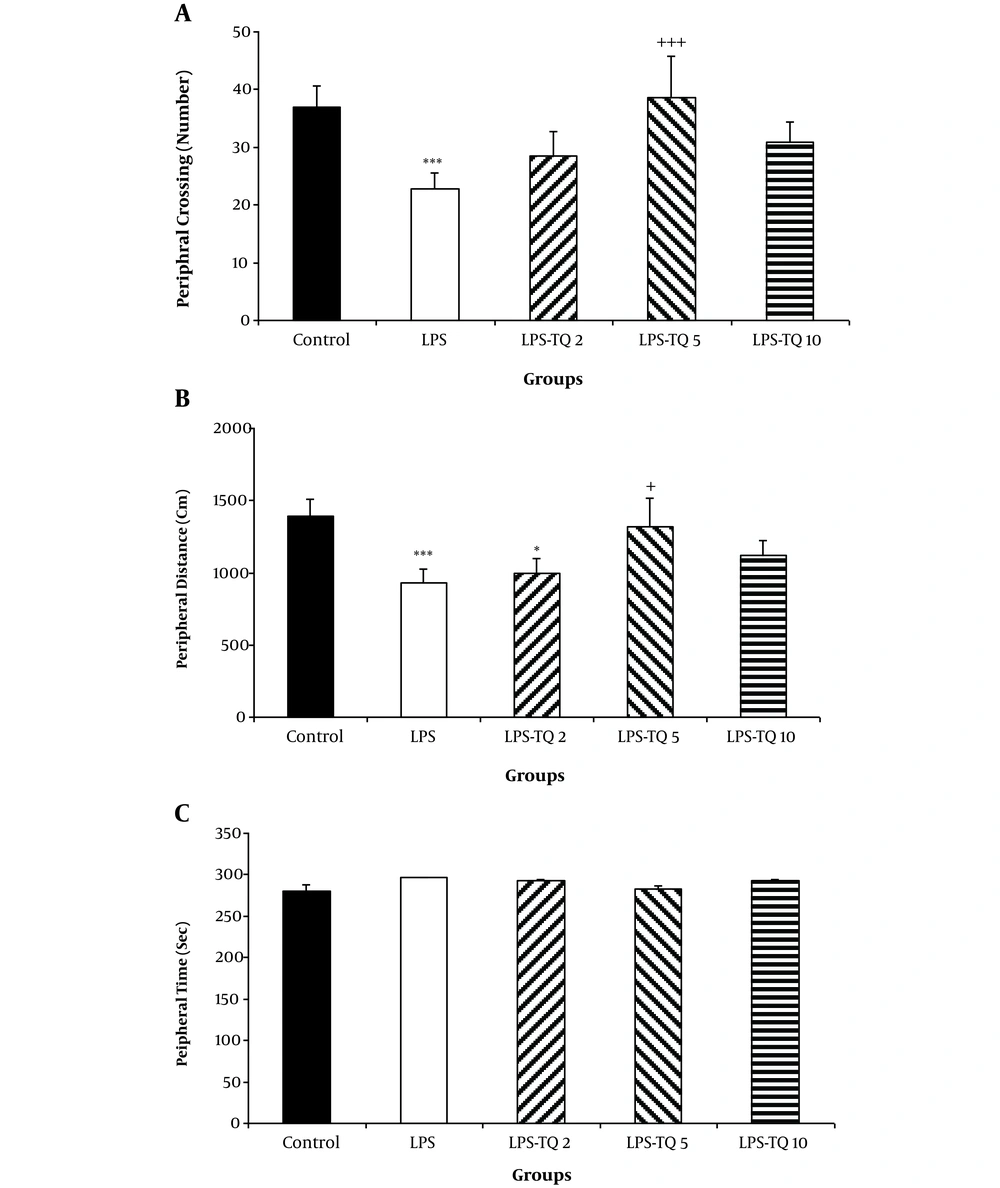
The results also revealed that the crossing number in the peripheral segment by the animals of the LPS group was fewer than that of the control group (P < 0.001; Figure 2A). According to Figure 2A, the peripheral crossing number by the rats of LPS-TQ 5 group was significantly more than the LPS group (P < 0.001; Figure 2A); however, no significant difference was observed between LPS-TQ 2, LPS-TQ 10 and LPS groups (Figure 2A). The peripheral zone distance in LPS and LPS-TQ2 groups was lower than the control group (P < 0.001and P < 0.05, respectively; Figure 2B). Peripheral zone distance in the LPS-TQ 5 group was more than the LPS group (P < 0.05; Figure 2B). However, no significant difference was observed in time spent in the peripheral zone between the LPS-TQ 2, LPS-TQ 10, and LPS groups (Figure 2B). No significant difference was seen between the groups when the peripheral zone time was compared between control and LPS groups as well as when the TQ-treated group was compared with the LPS group (Figure 2C).
A, Crossing; B, Distance; and C, Time in the peripheral zone in the open field test. Data are shown as the mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; ***, P < 0.001 compared to control group; +, P < 0.05; and +++, P < 0.001 compared to the LPS group.
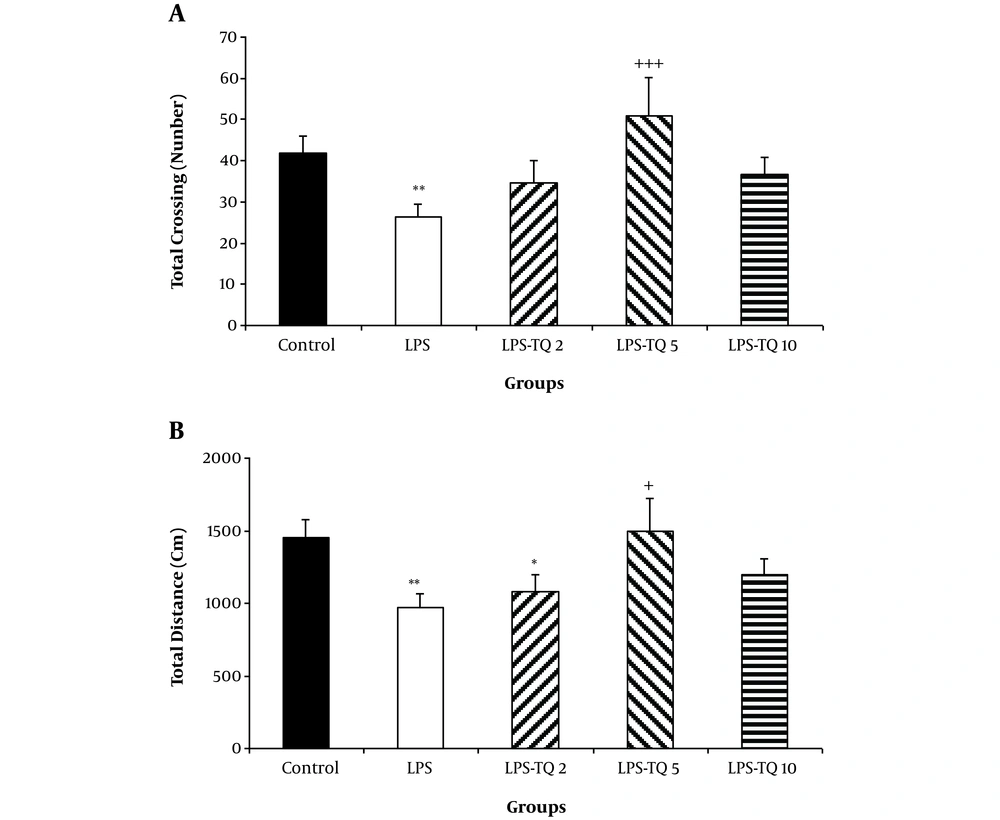
Additionally, the total crossing in the LPS group was lower than the control group (P < 0.01). The total crossing number in LPS-TQ 5 group was significantly more than the LPS group (P < 0.001, Figure 3A). No significant difference was seen between groups of LPS-TQ 2, LPS-TQ 10, and LPS. As shown in Figure 3B, the total distance in the LPS group was shorter than the control (P < 0.01) and LPS-TQ 5 (P < 0.05; Figure 3B) groups. But, there was no significant difference between LPS-TQ 2, LPS-TQ 10, and LPS groups. In addition, total distance in the LPS-TQ 2 group was lower than that in the control group (Figure 3B).
A, Total crossing; and B, Distance in the open field test. Data are shown as the mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 compared to control group; +, P < 0.05; and +++, P < 0.001 compared to the LPS group.
4.2. Elevated Plus-Maze
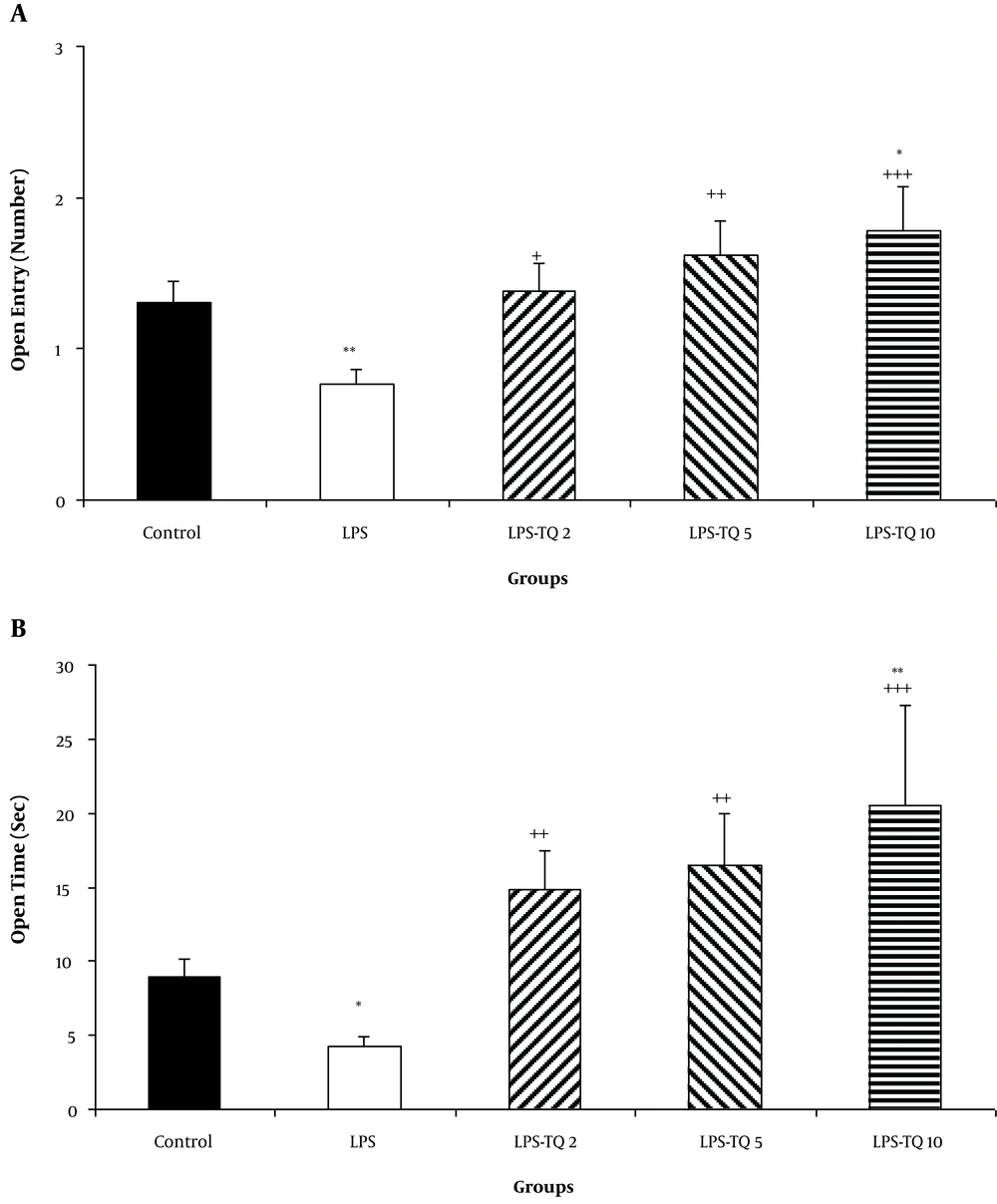
The open arm entries in the LPS group were lower than the control group (P < 0.01; Figure 4A). In all three TQ-treated groups, the open arm entries were more than the LPS group (P < 0.05 - P < 0.001; Figure 4A). The open arm entries in the LPS-TQ 10 group were more than the control group (P < 0.05; Figure 4A). The animals of the LPS-treated group also spent a long time in the open arm than the control ones (P < 0.05; Figure 4B). Treatment by all three doses of TQ prolonged the time spent in open arms (P < 0.01 - P < 0.001; Figure 4B). The open arm time in the LPS-TQ 10 group was more than the control group (P < 0.01; Figure 4B).
A, Open arm entries; and B, Time in the elevated plus-maze test. Data are shown as the mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; **, P < 0.01 compared to the control group; +, P < 0.05; ++, P < 0.01; and +++, P < 0.001 compared to the LPS group.
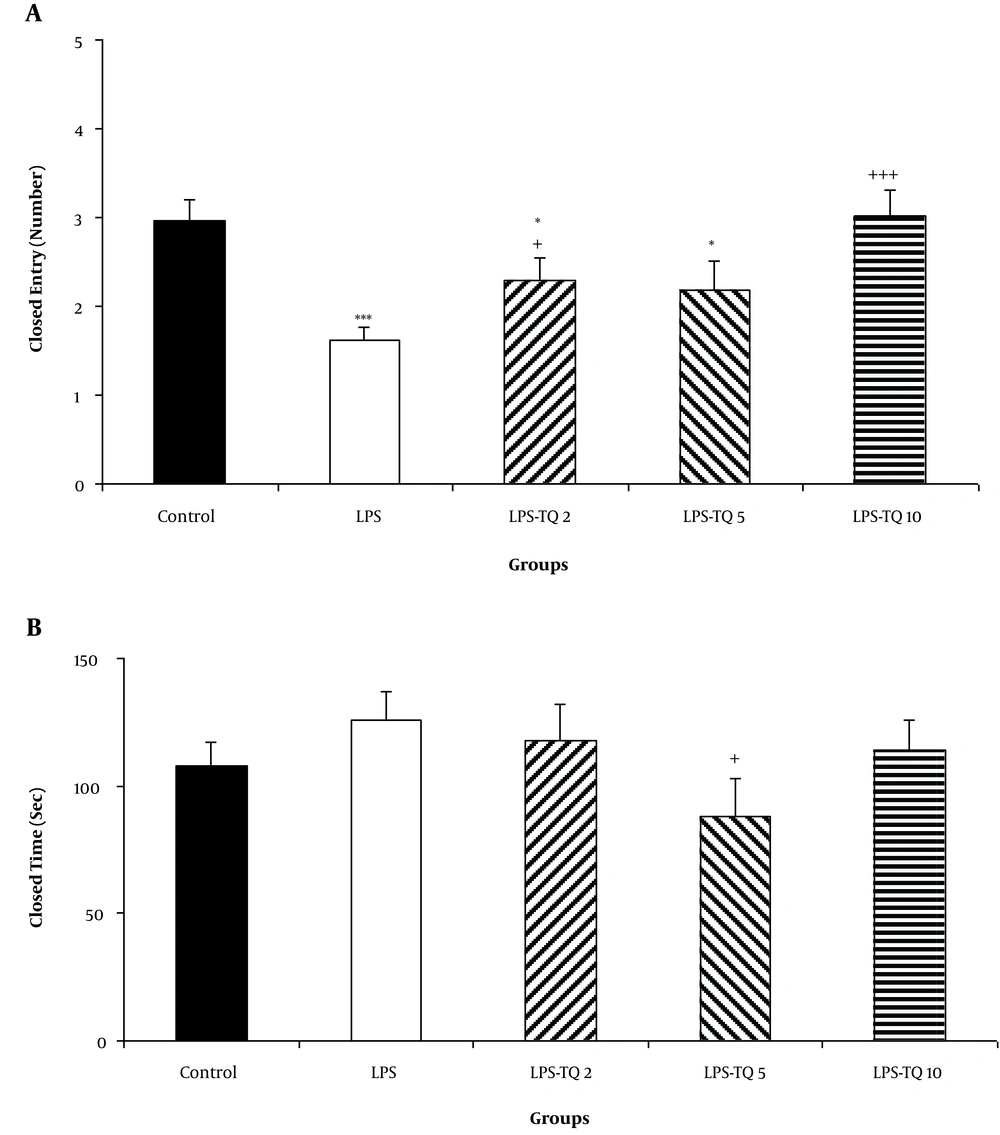
The results also showed that the animals of LPS, LPS-TQ 2 and LPS-TQ 5 groups were entered more frequently to the closed arm than the control ones (P < 0.001, P < 0.05, and P < 0.05 respectively; Figure 5A). No significant difference was observed when the entries to the closed arm were compared between LPS-TQ 5 and LPS groups, while this parameter in LPS-TQ 2 and LPS-TQ 10 groups was more than the LPS group (P < 0.05 - P < 0.001; Figure 5A). The time spent in the closed arm was no significantly different between the two control and LPS groups (Figure 5B). Compared to the LPS group, the LPS-TQ 5 group spent a lower time in the closed arm (P < 0.05; Figure 5B); however, there was no difference between the LPS-TQ 2, LPS-TQ 10, and LPS groups.
4.3. Forced Swimming Test
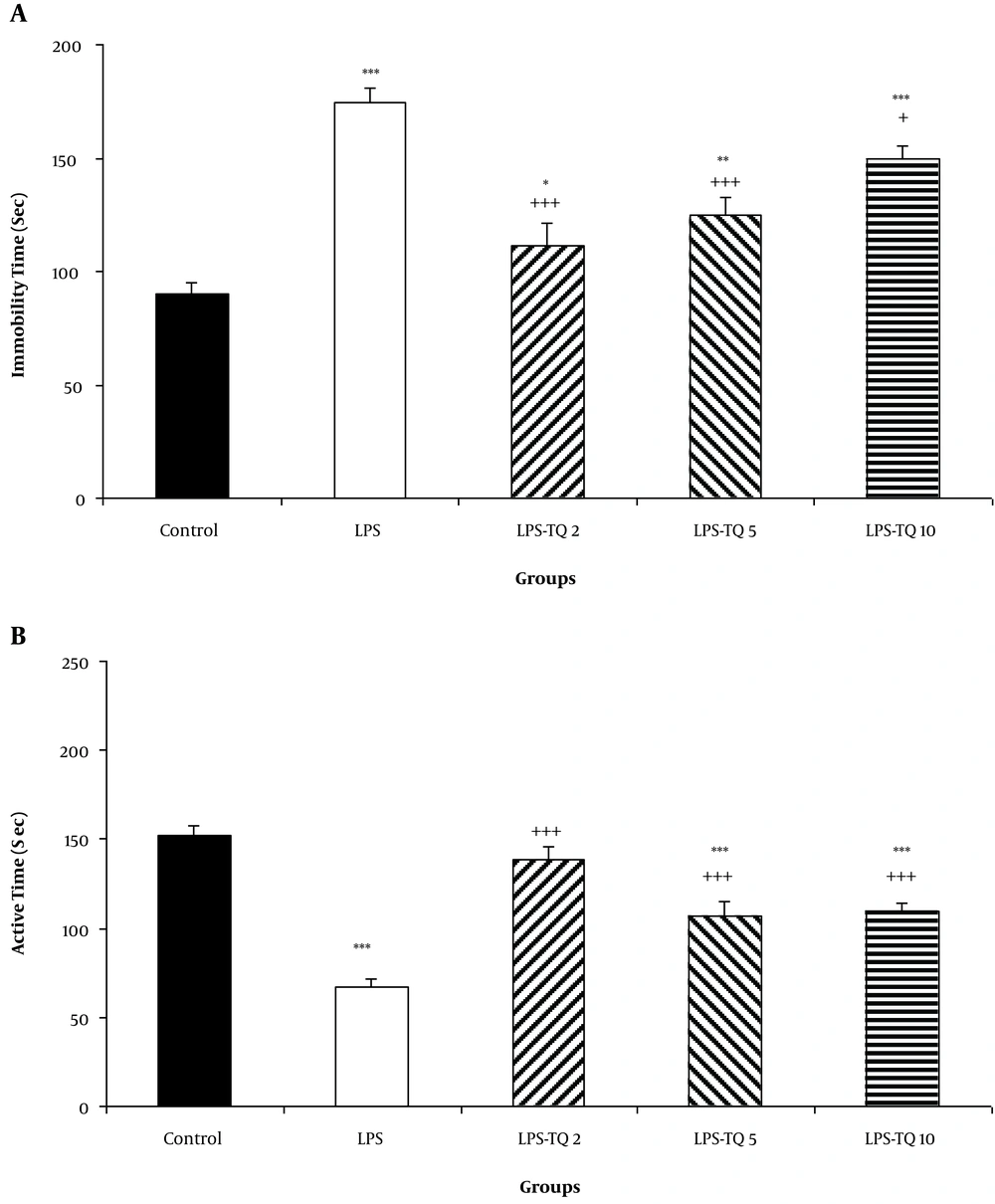
The results showed that LPS injection increased the immobility times in the LPS group compared to the control group (P < 0.001; Figure 6A). This parameter in all three TQ-treated groups was lower than the LPS group (P < 0.05 - P < 0.001; Figure 6A). Immobility times also in LPS-TQ 2, LPS-TQ 5, and LPS-TQ 10 groups were longer than that in the control group (P < 0.05 - P < 0.001; Figure 6A). Additionally, compared to the control group, LPS injection shortened the active times in the LPS group (P < 0.001; Figure 6B). However, this parameter in all three TQ-treated groups were more than the LPS group (P < 0.001; Figure 6B). The times also in LPS-TQ 5 and LPS-TQ 10 groups were shorter than that in the control group (P < 0.001; Figure 6B).
A, Immobility; and B, Active times in the forced swimming test. Data are shown as the mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to the control group; +, P < 0.05; and +++, P < 0.001 compared to the LPS group.
4.4. Blood-Brain Barrier Permeability
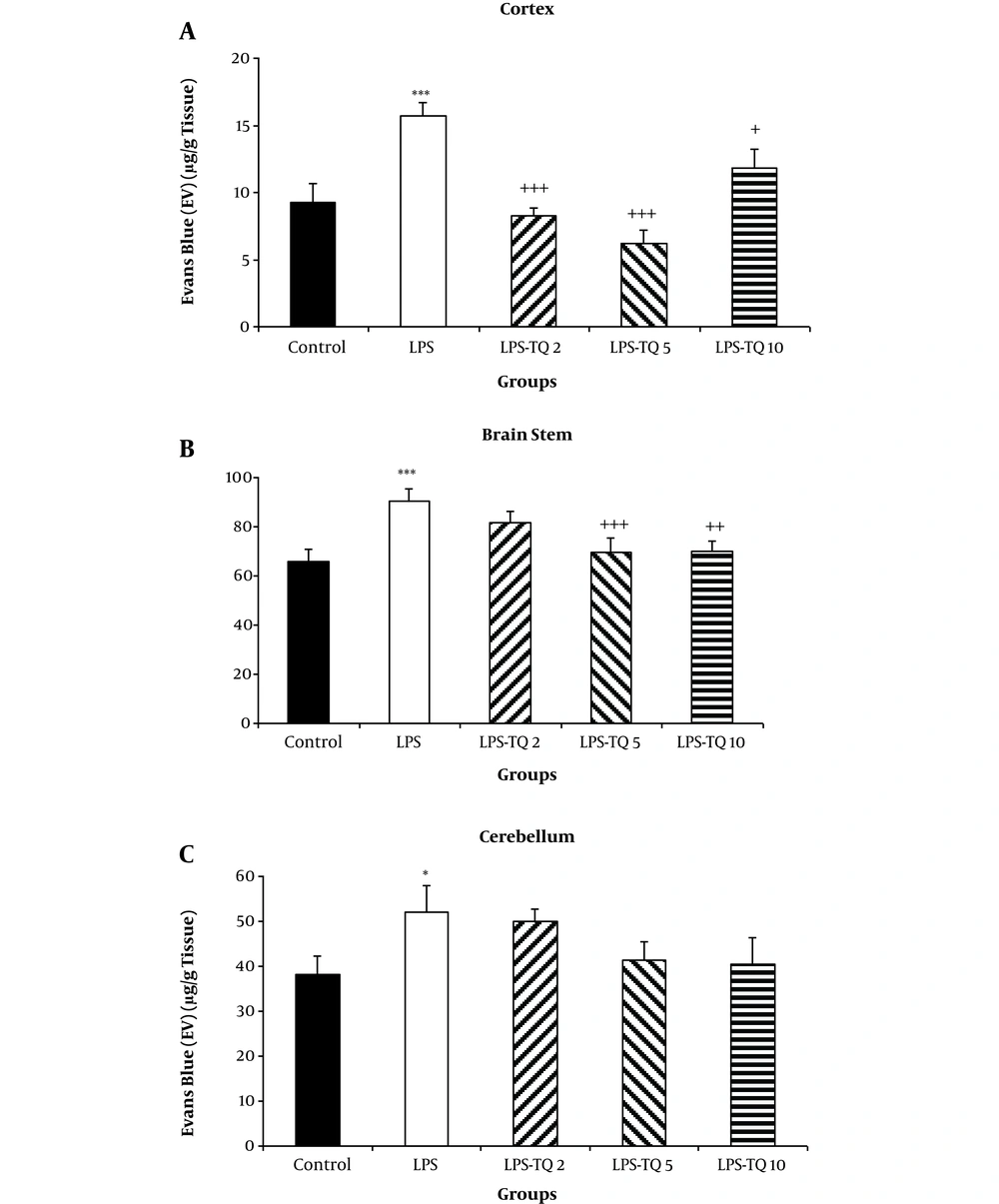
EB dye content in the brain tissues, including cortex, brain stem, and cerebellum of the LPS group was significantly more than the control group (P < 0.05 - P < 0.001; Figure 7A-C). Treatment by all three doses of the extract reduced EB dye content of the cortical tissues compared to the LPS group (P < 0.05 - P < 0.001; Figure 7A). The two highest doses of TQ also attenuated the EB content of the brain stem tissues (P < 0.05 - P < 0.001; Figure 7B). As Figure 7C shows there was no significant difference between the TQ-treated and LPS groups in the BBB permeability of cerebellum (Figure 7C).
EB dye content in the cortex (A), brain stem (B), and cerebellum(C). Data are shown as mean ± standard error of the mean (SEM) (number of animals in each group is 10). *, P < 0.05; ***, P < 0.001 compared to the control group; +, P < 0.05; ++, P < 0.01; and +++, P < 0.001 compared to the LPS group.
5. Discussion
In the current study, we showed that TQ was able to protect BBB and prevent the sickness behaviors induced by LPS in rats. Anatomical studies of BBB show that it is comprised of especially endothelial cells. These barriers are vital for normal physiological functions of the brain and spinal cord (1). Moreover, the BBB prevents free circulation of subtends between blood and CNS due to a high density of tight junction. This structure provides conditions for the proper functioning of the BBB (20). Studies show that some conditions affect this layer and change the permeability of the BBB (21), one of these conditions is inflammation (22). So inflammatory processes are known to disrupt BBB integrity (23, 24).
Inflammation is a physiological and primary response to various stimuli such as infection and tissue wounds. Inflammatory responses, on the one hand, stimulate the innate and acquired immune system and on the other hand, affect the epithelial duct as well as modify neuroendocrine status and behavioral changes, which lead to energy preservation such as increased sleep, lethargy, reduced appetite, and fever (25, 26). According to several studies, LPS, as a bacterial endotoxin and a powerful inducer of cytokine release, is frequently used to induce a common model for creating inflammation (23). Its effect is also known to cause neuronal inflammation (27). LPS leads to a release of NO, prostaglandins, and inflammatory cytokines such as IL-1, TNF-a, and IL-6 by activating macrophages through binding to toll-like receptor-4 (TLR4). In this condition, brain endothelial cells in the face of these materials lose their integrity (28-30). In this study, a higher EB dye content in the brain tissues of LPS-treated rats compared with the control ones confirms the destructive effect of LPS on BBB. It has been indicated that LPS induces BBB disruption through a cyclooxygenase (COX)-dependent pathway, possibly one that involves secretion of cytokines from the brain endothelial cells (31). In addition, experimental animal studies suggest that TNF-α alters the permeability of the BBB and due to a migration of leukocytes into the CNS by enhancing adhesion between leukocytes and BECs (32). On the other hand, TNF-α also induces NO generation, which later contributes to disruption of the BBB (33). It has been suggested that penetration of LPS to the BBB might be responsible for the events such as lethargy, fatigue, increased sleep, and reduced mobility and that collectively known as sickness behaviors (34).
In this study, TQ increased central crossing, central traveled distance, peripheral crossing, peripheral traveled distance, total crossing and total traveled distance in OF test. Considering all of these findings, accompanying protective effects of TQ against BBB permeability with the beneficial effects on sickness behaviors in LPS-induced model of rats might be suggested. Other studies also reported that TQ exerts its antioxidant and anti-inflammatory effects through inhibition of cytokines release (TNF-α, IL-1β). Studies show that TQ has also been able to suppress oxidative stress-induced neuropathy accompanying by a reduced level in nuclear factor kappa B activity in the brain and spinal cord (35, 36). In the present study, we did not compare the effects of TQ on BBB in different regions, including cortex, cerebellum, and brain stem, and the aim of this study was not such a comparison. However, at a glance, it seems that EB content in the cerebellum and brain stem tissues of the control group was more than that of cortex. We did not compare the effects of LPS on BBB between different parts of the brain. The effects of TQ have not also been previously evaluated but considering the previous studies, it seems that BBB is tighter in the cortex than the brain stem and the cerebellum (37-39); however, it needs to be more investigated in the future.
In this study, performances of the LPS-treated rats in EPM and OP tests also confirmed accompanying sickness behaviors with increasing of BBB permeability. Administration of LPS decreased the time spent in and the number of entries into the open arms of EPM and the time spent in a central area of apparatus, which might be considered a sign of anxiety-like behaviors in LPS-treated rats (16, 40). LPS also decreased the entries into the closed arm in EPM. The animals of the LPS group also had a lower crossing and a shorter traveled distance in the peripheral zone of apparatus. LPS also decreased the total traveled distance and total crossing number in the OF test. All of these findings confirm sickness behaviors induced by LPS injection (16, 40).
Medicinal plants have indicated considerable therapeutic effects possibly because of having several compounds with strong antioxidant and anti-inflammatory attributes (41). Owing to more adverse effects of synthetic antidepressants and on the other hand, expanding safe and effective agents from traditional herbs, researchers incline to use medicinal plants, and they found that a large number of antioxidants showed some beneficial effects on human health, as well as many of them such as NS, showed antidepressant-like effects (42). NS commonly known as the black seed is used as a natural treatment for some diseases. Researches have shown that the most effective treatment of NS is especially related to its constituent TQ (43, 44). It has been previously reported that TQ exerts an antidepressant-like effect by inhibiting the uptake of norepinephrine, serotonin, and dopamine (45). Perveen et al. (cited in Aquib) reported that TQ increased concentrations of 5 hydroxytryptamine (5-HT) and 5-hydroxy indole acetic acid (5-HIAA) in rats’ brains (46). Meanwhile, TQ is suggested to apply the antidepressant-like effect through antioxidant effects and protection against lipid peroxidation (47). Moreover, various studies have proved anti-inflammatory effects of TQ (46, 48). In this study, all doses of TQ decreased immobility times, while increased active time in FST, which confirms its anti-depression-like effects in an LPS-induced model in rats. As was mentioned above, BBB impairment has an important role in behavioral impairments due to neuroinflammation. Therefore, besides other mechanism(s) which were explained, protection against BBB rupture was examined as a mechanism for the beneficial effects of TQ in the brain. The results showed that EB dye contents of the brains of TQ-treated rats were lower than that of LPS-treated ones. EPM is a well-known experiment to assess anti-anxiety compounds. The compounds with anti-anxiety effects remarkably increased the entries into, and time spent in the open arms (49, 50). In this study, all doses of TQ increased the open arm entries and the time spent in the open arms of EPM, which might be considered its anti-anxiety effects. Supporting, TQ has previously shown to have anti-anxiety-like activity possibly through modulation of NO and gamma-aminobutyric acid (GABA) (51). The FST is broadly utilized for screening substances for potential antidepressant effects (52, 53). In this study, in addition to increasing BBB permeability, LPS injection increased immobility time while, decreased active time in the FST, which might be considered a depression-like behavior (54, 55) as well as a sign of sickness behavior induced by LPS in rats (16, 40). Accordingly, Hines et al. (56) also found that after LPS injection, the BBB breakdown occurred, which comforted the migration of macrophages into the brain; thus worsened neuroinflammatory and resulted in sickness behavior. Other studies also demonstrated that exposure to LPS reduced serotonin and noradrenaline levels in the brain and caused a change in emotion (57). Previously, it has been reported that LPS induces neuroinflammation by increasing BBB permeability, leading to neuronal damage, especially ineffective brain areas in emotion, including the limbic system (10). It has also been suggested that BBB disturbance may contribute to some neuropathology conditions such as multiple sclerosis, epilepsy and Alzheimer’s disease (58). On the other hand, inflammatory response in the brain has been found to create huge amounts of reactive oxygen species, which prompt oxidative stress and exacerbate the BBB damage (59, 60).
In conclusion, the results of this study showed that administration of LPS induced sickness behaviors in rats, which reflected impaired performances of the rats in FST, OF, and EPM. The sickness behaviors were accompanied by BBB impairments. TQ protected BBB disruption induced by LPS and also improved performances of the rats in behavioral tests. In the present study, considering the results of OF test and EPM in which TQ improved performances of the rats compared to the control group, it seems that TQ by itself has an anti-anxiety effect. Considering these results, besides other mechanism(s) which have been previously explained, protection against BBB permeability, as a possible mechanism for protective effects of TQ against sickness behaviors induced by LPS, is suggested; however, it needs to be more investigated.