1. Background
Since its discovery, nanoparticles (NPs) have attracted the attention of scientific communities. In the past few years, NPs have been extensively used in different areas, such as electronics, catalysis, microscopy, development of antimicrobial agents, and enhanced photocatalytic degradation of organic dyes (1). Generally, NPs are synthesized using chemical processes involving hazardous chemicals, reducing agents, and capping agents under controlled conditions. However, they are expensive, which may limit their widespread application in health and environmental fields.
Recently, several researchers have reported green synthesis of NPs, using microorganisms or plant-based products (2-10). Among different types of NPs, silver NPs (AgNPs) are reported to be synthesized, using different plant-based constituents for different applications, including antimicrobial, antifungal, antioxidant, and environmental purposes (11, 12). These green-synthesized NPs are as active as their chemically synthesized counterparts; additionally, they are rapidly synthesized and are environmentally friendly. Plant-based NPs are even easier to produce compared to microbe-based NPs, as they do not require preparation of any nutritive media for microbe cultivation or maintenance of sterility during cultivation.
Plants and their different parts (e.g., leaf and fruit) contain several reducing, oxidizing, capping, and stabilizing constituents, such as minerals, organic acids, phenolics, proteins, and enzymes, which promote synthesis of NPs (13). Pomegranate (Punica granatum) is one of the most important crops cultivated in Oman. This fruit has been widely used in culinary products and cuisines worldwide with medicinal values.
The pomegranate peel is an agrowaste, which accounts for approximately 60% of the weight of pomegranate fruit. The pomegranate peel extract (PE) is also known for its high-nutrient content, consisting of minerals (e.g., potassium), vitamins, phenolics, flavonoids, and antioxidants (14, 15). In the present study, we used and characterized pomegranate PE for synthesis of AgNPs from silver nitrate (AgNO3) and used it for photocatalytic degradation of methylene blue (MB) dye.
2. Objectives
In this study, we evaluated the possibility of AgNP synthesis using aqueous pomegranate PE and examined its application in photocatalytic dye degradation. The synthesized AgNPs were characterized by various analytical tools and used for degradation of MB dye under solar irradiation.
3. Methods
3.1. Materials
Fresh pomegranates were purchased from local farms, while the rest of the chemicals were purchased from Sigma, USA; all the chemicals were extra pure and of an analytical grade. Ultrapure water (18.2 MΩ) was used for all the experiments.
3.2. Collection and Preparation of Pomegranate PE
Fresh pomegranates were purchased from the local farms of Al-Jabal Al-Akhdhar, a mountain range in Oman. After the fruits were washed with ultrapure water and cut, the arils were separated from the peels. The peels were chopped into small pieces, added to ultrapure water (1% w/v), boiled at 70 - 75°C for 15 minutes, and left to cool down. They were then filtered through Whatman® qualitative filter papers (grade I; Sigma-Aldrich, USA) and used for further experiments.
3.3. Biosynthesis and Characterization of AgNPs
To prepare AgNPs, pH of freshly prepared PE was adjusted to 8.0, and then, silver nitrate (final concentration, 1 mM AgNO3) was added and completely dissolved at ambient temperature (21 ± 1.5°C). PE and PE-mediated AgNPs were analyzed immediately and at different intervals for 48 hours via UV-VIS spectrophotometry (Nanodrop 2000c, Thermo Scientific, USA) at room temperature in a wavelength range of 190 - 750 nm.
The synthesized PE-mediated AgNPs were further analyzed for particle size distribution and zeta potential (Nanotrac Wave, Microtrac, USA). X-ray diffraction (XRD) measurements were performed using a powder XRD method in a PANalytical-X'Pert PRO diffractometer with a Cu-Kα source (1.5405 Å), based on standard procedures. The morphology and elemental composition of PE-mediated AgNPs were investigated through energy-dispersive X-ray (EDX) analysis, using an Oxford Inca/Aztec EDS instrument, coupled with a JSM-7600F field-emission scanning electron microscope (FE-SEM; JEOL, Japan). The infrared spectrum was also recorded by a Fourier-transform infrared (FTIR) spectrometer (Cary 630; Agilent Technologies, USA).
3.4. Photocatalytic Activity of PE-Mediated AgNPs
The photocatalytic activity of biosynthesized PE-mediated AgNPs was evaluated by degradation of an organic dye (MB) under direct sunlight. For this purpose, 10 mg/l of MB dye stock solution was prepared in ultrapure water, and about 10 mg of biosynthesized AgNPs was added to 100 mL of MB dye solution. A control was also maintained without the addition of PE-mediated AgNPs. The solution was exposed to sunlight irradiation (outside temperature, 35 - 40°C), and the progress of reaction was monitored via UV-VIS spectroscopy. The values of absorption maxima (λmax = 660 nm) were compared with that of MB at different intervals after filtration.
4. Results
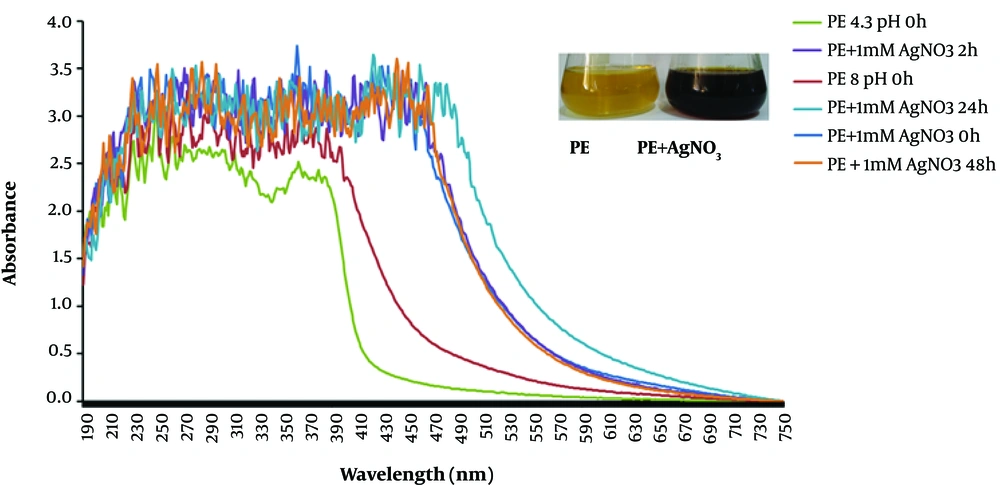
AgNPs were synthesized using pomegranate PE as a reducing agent at ambient temperature. The color of the reaction mixture turned from pale yellow to dark brown, indicating AgNO3 reduction within five minutes, besides biosynthesis of AgNPs. The broad surface plasmon resonance (SPR) peak appeared at a wavelength range of 432 - 442 nm after five minutes of reaction time (Figure 1); the figure insert shows the color of PE (pale yellow) and PE + AgNO3 (dark brown). Other reports have indicated AgNP synthesis within 10 - 30 minutes, using peel or plant extracts, based on SPR peaks in the mentioned wavelength (16-18). The broad peak of UV absorbance at 432 - 442 nm is in accordance with previous reports (1, 19, 20).
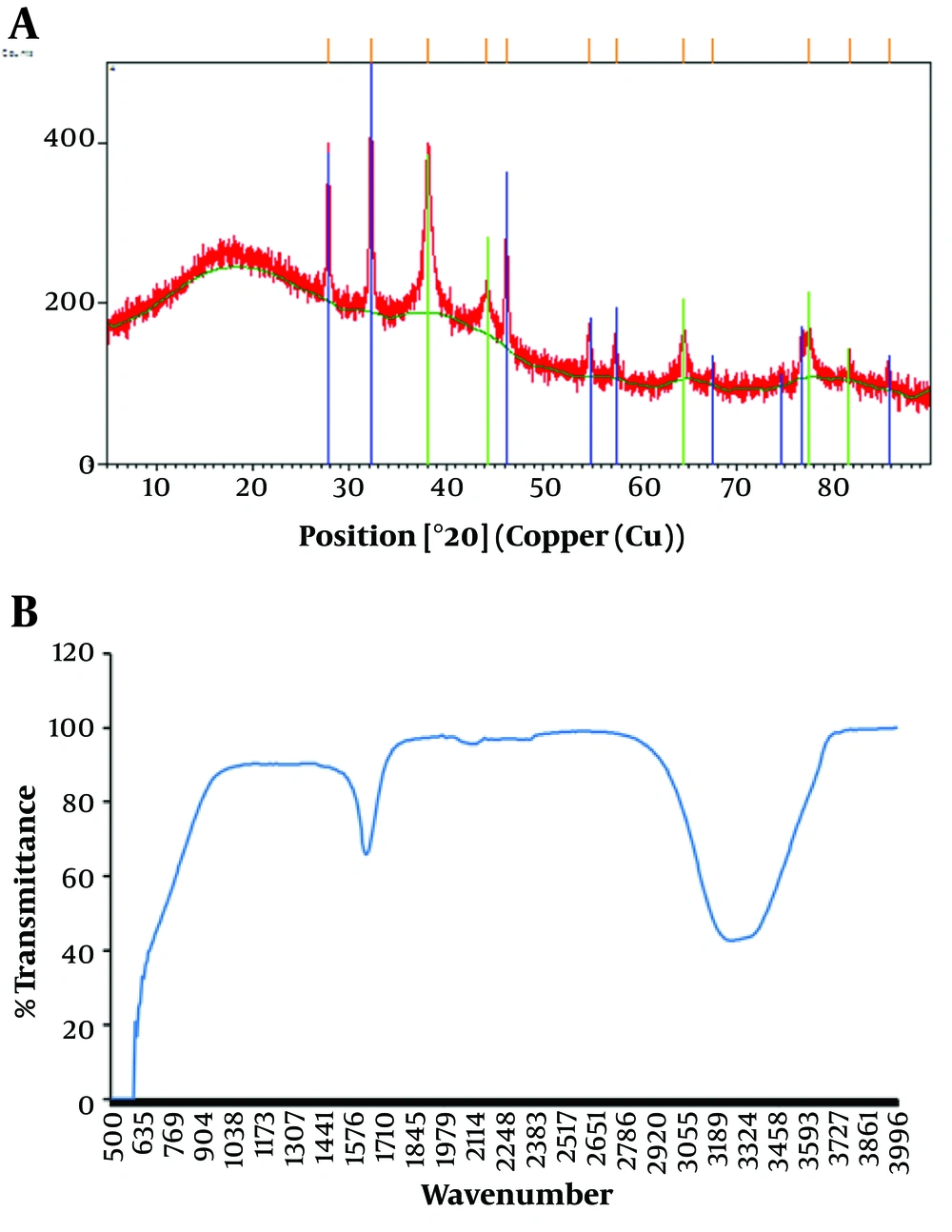
Earlier reports indicate that different phytoconstituents, such as polyphenolics in the pomegranate PE, could play a key role in the conversion of the ionic form of silver to the nano form (19). The XRD pattern of biosynthesized AgNPs is shown in Figure 2A. The diffraction peaks at 2θ = 38.18°, 44.31°, 64.61°, and 77.50° were assigned to the corresponding diffraction signals (111), (200), (220), and (311), respectively, which indicate the cubic crystal structure of AgNPs (JCPDS No., 04-0783).
The results suggest that AgNPs were crystalline in nature. Other minor peaks were also observed, which might have emerged due to impurities and some unutilized AgNO3 in the samples. Karuppiah and Rajmohan (17) reported similar results for AgNPs synthesized by Ixora coccinea leaf extract. The FTIR peaks (Figure 2B) further supported PE-mediated AgNP formation. The FTIR spectrum showed major absorption peaks at 3286 cm-1, which corresponded to -OH stretching of phenolic and carboxyl groups, respectively. Furthermore, the absorption band at 1636 cm-1 was related to the presence of N-H bending of primary amines (11, 19).
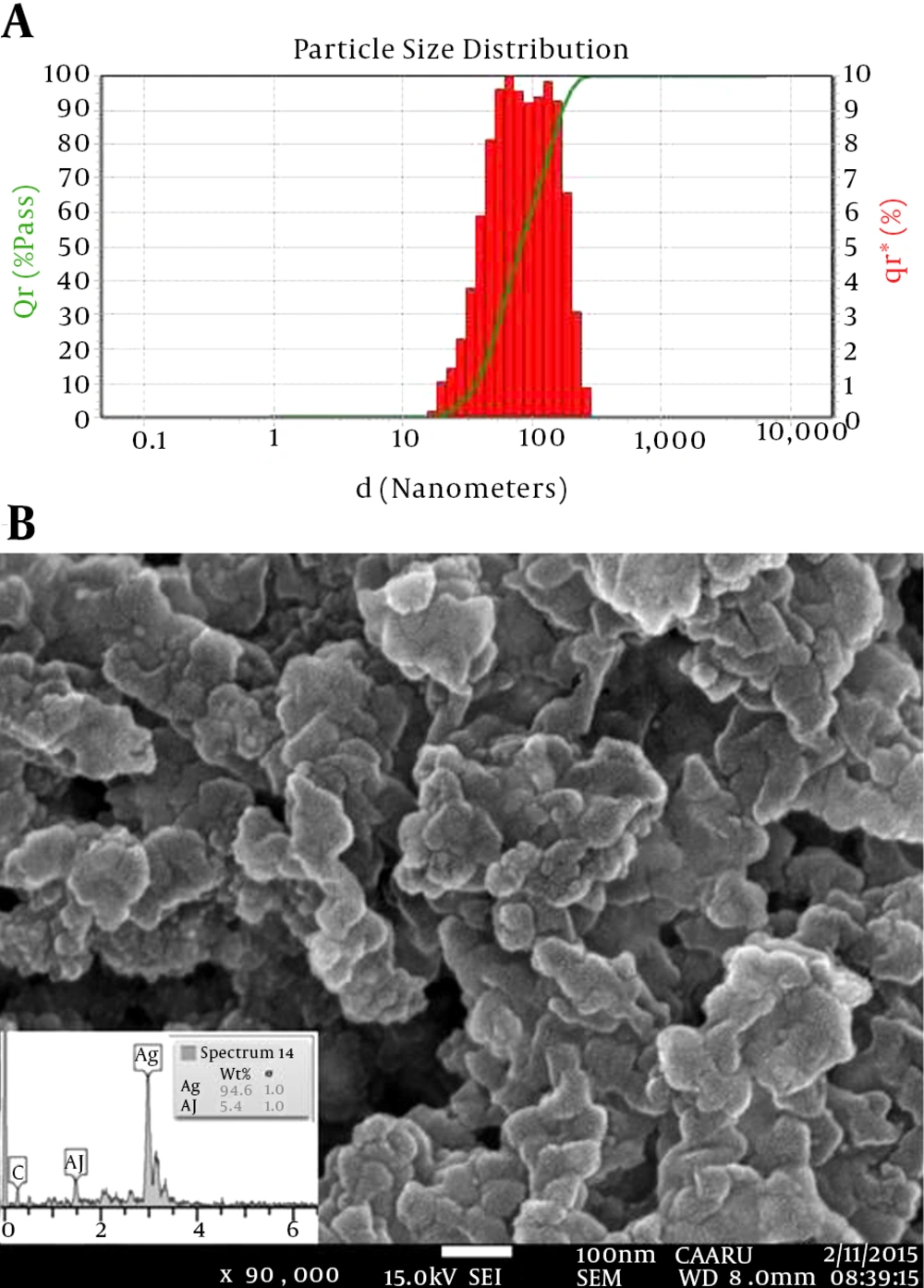
The FTIR spectra indicated that the phytoconstituents of pomegranate PE, such as polyphenols, might be involved in the process of AgNP biosynthesis. The size distribution of PE-mediated AgNPs is presented in Figure 3A. According to the figure, it can be inferred that the average particle size distribution of PE-mediated AgNPs was 57.7 - 142.4 nm, and the corresponding zeta potential was -68.93 mV, suggesting the high stability of AgNPs. The high negative potential value could be attributed to capping of polyphenolic constituents in the pomegranate peel (19).
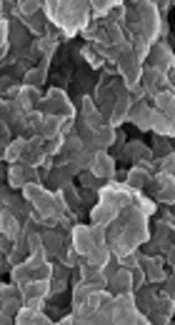
In addition, Padalia et al. (18) reported a zeta potential of -27.63 mV for Tagetes erecta flower-mediated AgNPs. The FE-SEM image of PE-mediated AgNPs (Figure 3B) at high magnifications showed them to be spherical, with a great agglomerate size range (60 - 150 nm). The elemental EDX analysis revealed strong peaks in the silver region (3 keV) at around 94.6 wt% and confirmed the formation of AgNPs (Figure 3B).
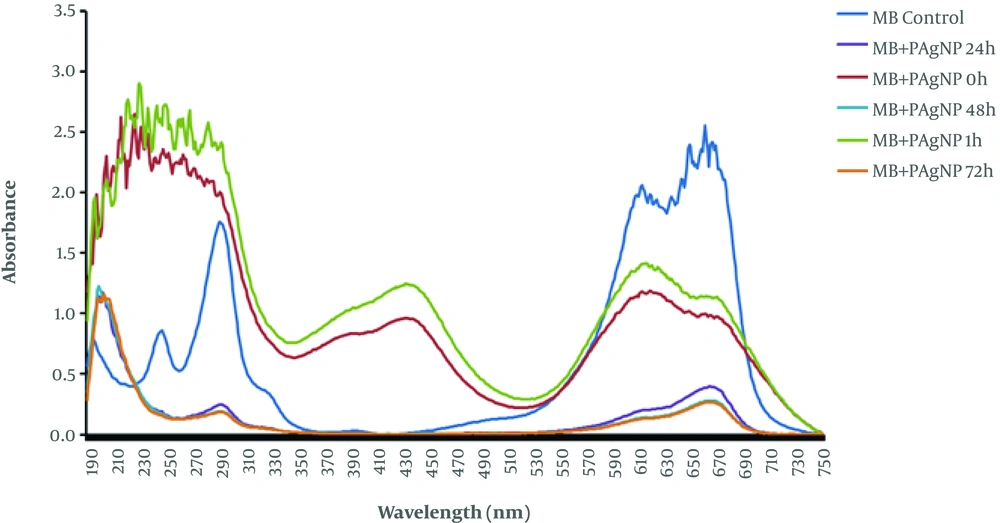
The catalytic activity of biosynthesized PE-mediated AgNPs was analyzed for degradation of an organic dye, MB, under solar irradiation. MB degradation was initially identified by visible color changes immediately after adding PE-mediated AgNPs. The deep-blue color of the dye changed into light blue-green within 30 - 60 minutes of incubation with PE-mediated AgNPs under sunlight. The absorbance of MB (2.557) was observed at 660 nm, which was reduced to almost 55 - 60% within one hour and degraded to almost 89% by 48 - 72 hours (Figure 4).
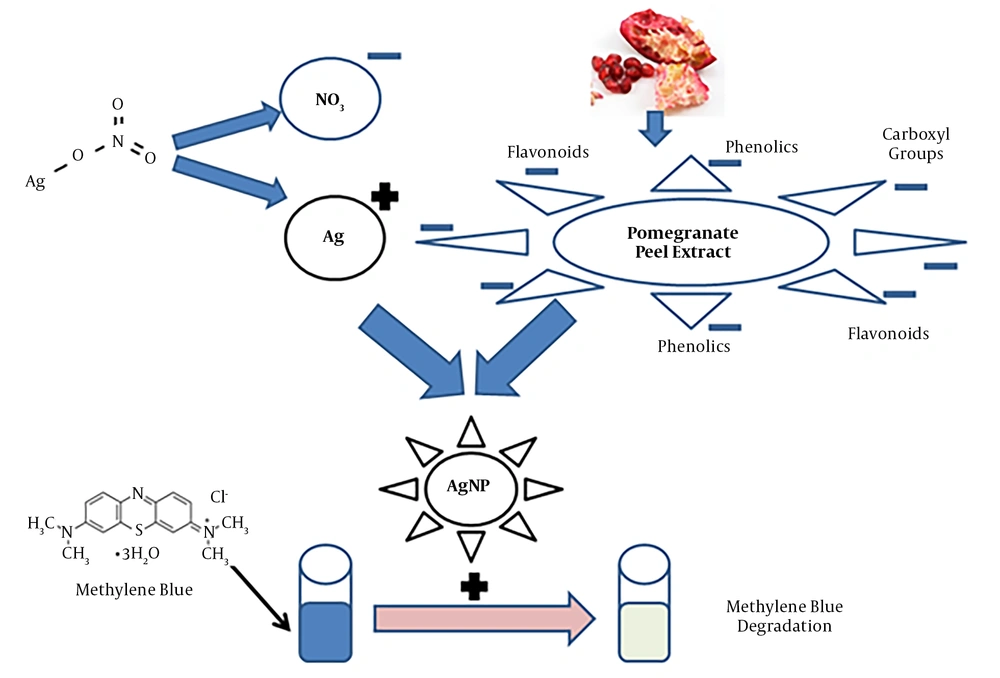
Figure 5 presents the proposed mechanism of AgNP biosynthesis in the presence of PE and photocatalytic effects of synthesized AgNPs. Kumar et al. (16) explained that this reduction in the absorbance peak of MB dye in the presence of NPs is related to the electron relay effect.
4.1. Conclusion
AgNPs could be synthesized rapidly and successfully within five minutes, using pomegranate PE under ambient conditions. The polyphenol molecules in the pomegranate PE served two purposes as both reducing and stabilizing agents. The synthesized AgNPs showed effective photocatalytic activity.