1. Background
The Teucrium polium (T. polium) is used in traditional medicine to cure various diseases (1). It is well established that T. polium in medical sciences contains inappropriate effects, for example, hepatitis, changes in the kidney, and allergic response. It is not safe to use T. polium during pregnancy or lactation (2, 3).
Herbs may also induce vascular injury and affect embryonic vascular development (4). Angiogenesis is the genesis of new vessels from primary vasculature and is a critical step in embryo survival and development. Several growth factors are recognized to be crucial for regulating angiogenesis. The vascular endothelial growth factor A (VEGF-A) is generated via ectodermal and mesenchymal tissues (5).
Assessment of the pathological activity of substances needs a suitable animal model. Use of the extra-embryonic membrane (EEM) of the chicks has been promoted to evaluate the effects of angiogenic/anti-angiogenic compounds (6).
2. Objectives
Although increasing consumption and production of various compounds from T. polium have happened in some areas, few studies have been conducted about this plant's side effects on the vascular plexus. Furthermore, the precise pathway that the herb affects vessel formation and development is not thoroughly investigated.
The paper was performed to answer the issue:
(i) Does T. polium alter the early development of the EEM-vasculature?
(ii) Does T. polium alter VEGF-A expression in the extra-embryonic vascular plexus?
A chick embryo model was used to answer the above questions. Various instruments and software were also used to analyze the vascular plexus of the EEM to demonstrate the anti-angiogenic activity of the T. polium. The data were combined by polymerase chain reaction (PCR) assay to evaluate various genes' expression.
3. Methods
3.1. Eggs
Fertile chicken eggs (Ross 308) (mean weight: 50 ± 0.6 g) were prepared from the Mahan Broiler company, Kerman, Iran.
3.2. Herbal Plant Extract
Teucrium polium (Cat. No. 232-33B) was prepared from the medicinal plants' Company, Kerman, Iran, in October 2015 and verified at the Department of Pharmacy of Kerman University of Medical Sciences in Iran. The herbal extraction was made by the Soxhlet method at 100.0 g for four h in 1000 mL of water/ethanol 80/20 v/v (7). The extraction yields 58% (w/w), calculated per weight of primary material.
3.3. Embryo Treatment and Image Acquisition
Chicken eggs were incubated in a commercial incubator (Damavand Company. CLP-SH, Rasht, Iran) at 60% humidity and 37°C. A pinhole was created in the shell after 24 h of the incubation period. Chicken eggs were injected with 50 μL T. polium extract or PBS as a sham group. The eggs were also re-injected at 24 and 48 h later. This treatment method has been described previously (8, 9). After injection, the pinpoint hole was closed with paraffin. On the fourth day of the incubation, the dead embryos were excluded. Finally, the injected eggs were classified into three groups: group 1 (n = 10): PBS-eggs (control), groups 2 (n = 10) and 3 (n = 10): herbal extract eggs, that eggs were injected with the T. polium extract at dosages of 3 (150 µg/50 µL) or 6 (300 µg/50 µL) mg/kg egg weight, respectively. A 25 × 25 mm opening was made in the shell to imaging the EEM vessels using a microscope (Luxeo; Labomed, CA, USA). The experimental assay was performed based on the European Ethical Guidelines of Animals in researches.
3.4. Vascular Branching Pattern Analysis
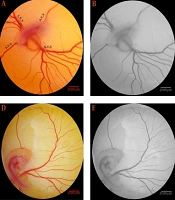
The computerized analysis was performed using image analysis software, such as MATLAB® (Matlab R2015a; Mathworks, Natick, MA, USA), ImageJ version 1.48 software, and Digimizer version4.3.0. First, a specific field was selected on images, and a 315 mm2 area, including 2,987 × 2,987 pixels, was extracted into the right-lateral vitelline vein (Figure 1A, D and G). The pictures were changed to a format of 8-bit, and the schematic structure of the vessels was processed (Figure 1B, E, and H). Finally, the color level was reduced to black and white and transformed into skeletonized pictures (Figure 1C, F, and I) to present the images' structural pattern. Vascular branching patterns were investigated for vessel area changes, total vessel length, vascular branching, and lacunarity (10-12). Lacunarity indicates areas without any vessel branches.
The vascular plexus of the day four embryos are presented to illustrate the image manipulations required for vascular branching pattern analysis. The images are captured from the embryo of the control (a - c) and Teucrium polium at dosages of 3 (d - f) or 6 (g - i) mg per kg egg-weight. (a, d, and g) A particular area, 315 mm2 containing 2987 × 2987 pixels, was identified at the right-lateral vitelline vascular plexus. (b, e, and h) The extracted areas were converted into an 8-bit format. (c, f, and i) The vascular branching pattern was ascertained from the skeletonized pictures.
3.5. Morphometric Analysis of Capillary Density
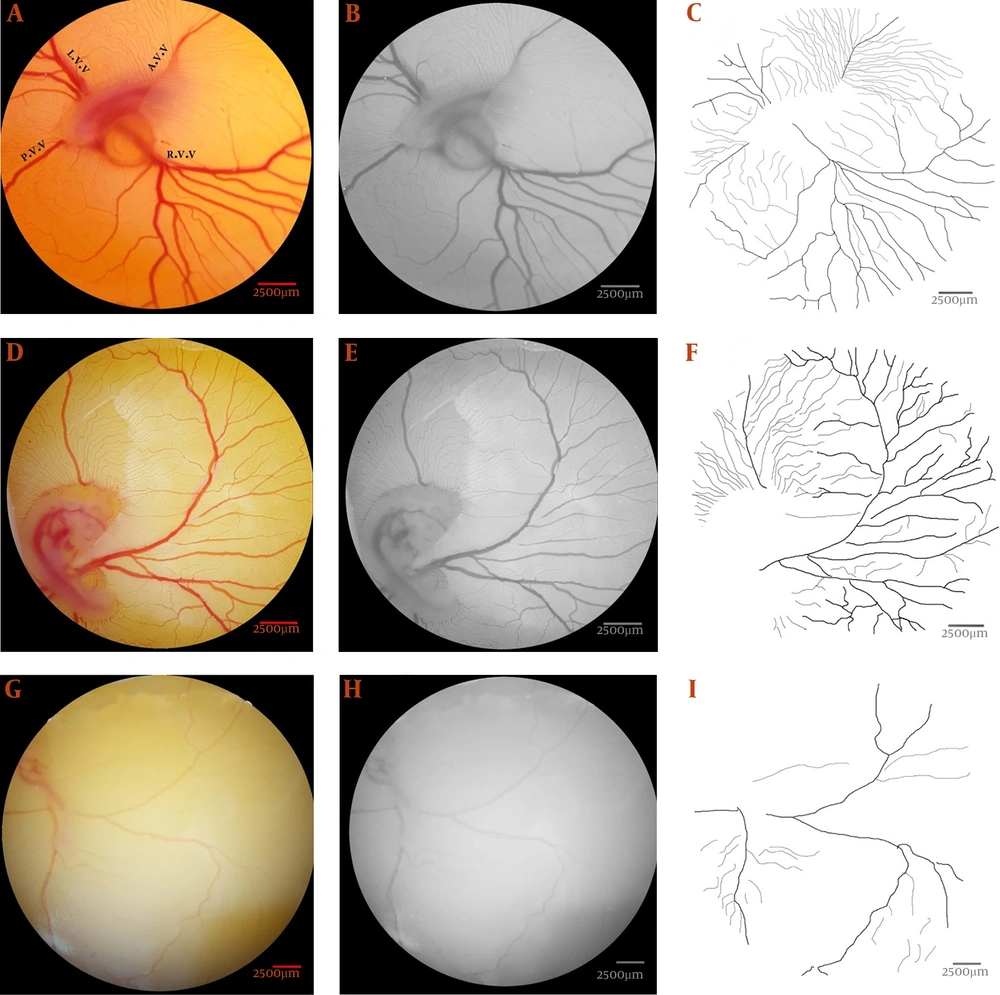
A defined field inside the right-lateral vein was drawn (Figure 2A). The selections were converted to black and white (Figure 2B), and the fields without any vasculature have been specified. Five fields were considered, and the percentage of the fields, including black color, selected (Figure 2C). The black pixels are blood in the basic images. The mean of all fields considered as mean capillary area (MCA) (13).
The mean capillary area (MCA) quantified from the chick's extra-embryonic membrane at day 4 of the incubation period. (a) The defined area inside the right-lateral vitelline vascular plexus. (b) The selection has been converted to a binarized image. (c) Five areas (arrows) without any branch vessels are selected, and the percentage of the areas containing black pixels was calculated for quantification of the MCA. The black pixels of the image indicate the red color, or blood, in the original image.
3.6. Effect of Teucrium polium on the Expression of VEGF-A
The expression of the VEGF-A gene was determined by real-time PCR (qPCR) assay. At the first step, total RNA was extracted via the RNeasy mini kit (Qiagen, USA), based on the standard instrument. The concentration of RNA (ng) and purity (260:280 nm) determined using NanoDropND-1000 spectrophotometer (NanoDrop Wilmington, USA). The cDNA was made using the TaKaRa Prime Script™ RT reagent kits (Takara Biology, Japan), and transcription assay was done by 500 ng total RNA. The real-time qPCR reaction was performed duplicated with the Rotorgene (Corbett, Australia) via an SYBR Green protocol (Takara Biology, Japan). The primers are presented in Table 1. At first, a holding step for 1 min at 95°C was made followed by 40 cycles of amplification (10 s with 95°C, 15 s with 60°C, and 20 s with 72°C).
| Gene (Gallus Gallus) | Sequence (5′ – 3′) | Product Size (Bp) |
|---|---|---|
| VEGF-A | 86 | |
| Forward | caattgaga ccc tggtgg ac | |
| Reverse | tct cat cag aggcacacagg | |
| GAPDH | 176 | |
| Forward | cctctctggcaaagtccaag | |
| Reverse | ggtcacgctcctggaaga ta |
3.7. Statistical Analyses
The statistical assay was done via the SPSS program. One-way variance and Turkey's test were applied used to analyze the parameters. The P-value of < 0.05 was significant data.
4. Results
4.1. Vascular Branching Pattern
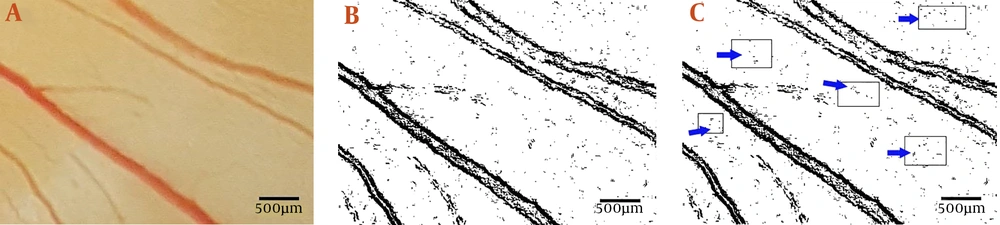
At the time of analysis, the stage of embryonic growth was 22 – 24 HH (Hamburger–Hamilton). The vascular plexus was around the chick embryos in the control group (Figure 3A). An altered vascular plexus performed around the embryos in the 6 mg/kg egg weight T. polium group. Vascular disruption is demonstrated as a decrease in branching (Figure 3B).
Embryonated eggs were treated three times at 24, 48, and 72 h of the incubation period. (a) Control embryo with normal extra-embryonic membrane vasculature is seen. (b) Embryonated egg received Teucrium polium extract at the dosage of 6 mg per kg egg-weight. Vascular disruption is demonstrated by decreased branching. A.V.V., anterior vitelline vein; L.V.V., left lateral vitelline vessel; P.V.V., posterior vitelline vein; R.V.V., right lateral vitelline vessel.
The analysis of the embryo vasculature due to the T. poliumt treatment is seen in Table 2. The T. polium disturbed the embryo vasculature that received high herb dosage. The vessel plexus parameters decreased in the group 3 embryos compared with the controls (P < 0.05), whereas lacunarity increased. The vascular pattern of the group 2 embryos was not significantly different from the control group.
| Parameters | Group | ||
|---|---|---|---|
| Control | Teucrium polium | ||
| Dosage (mg per kg egg-weight) | 3 | 6 | |
| Vessels area (%) | 53.3 ± 1.11 A | 52.72 ± 1.22 A | 28.73 ± 2.21 B |
| Total vessels length (Pixel) | 8722.81 ± 1.92 A | 8454.72 ± 2.23 A | 3923.53 ± 2.88 B |
| Vascular branch | 182 ± 2.62 A | 179 ± 2.52 A | 62 ± 3.25 B |
| lacunarity | 0.37 ± 0.17 A | 0.37 ± 0.19 A | 0.91 ± 0.35 B |
aValues are expressed as mean ± standard error of the mean.
bIn each row, different capital letters (A-B) in superscript show significant difference between groups (P < 0.05).
4.2. Capillary Density
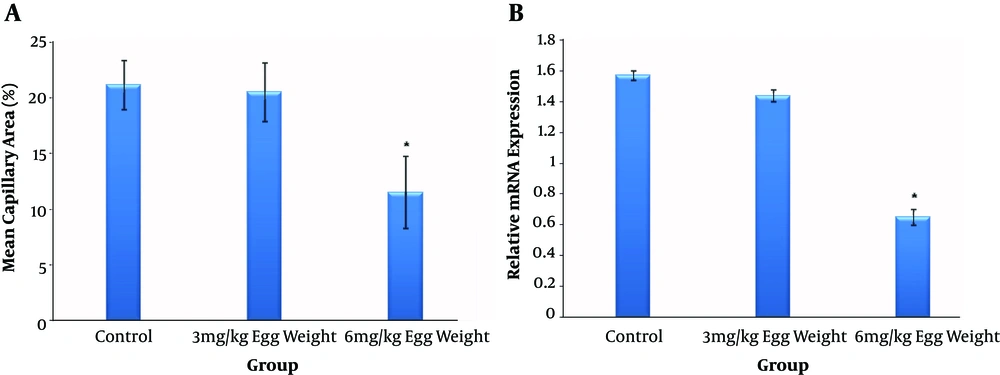
The results of the T. polium treatment on the EEM is presented in Figure 4A. A significant reduction in MCA was seen in treated embryos of group 3 (control, 21.22 ± 2.21; group 2, 20.58 ± 2.63; group 3, 11.53 ± 3.23; P < 0.05). Statistical results showed that the embryos injected with a larger dosage of T. polium showed MCA reduction with a dosage-dependent approach.
Effect of Teucrium polium extract on the early embryonic angiogenesis using the mean capillary area (MCA) quantification. Data are presented with comparison between control (n = 10) and Teucrium polium extract at dosages of 3 (n = 10) or 6 (n = 10) mg per kg egg-weight. The MCA was reduced in the Teucrium polium-treated group of 6 mg per kg egg-weight (error bars show standard error of mean; *P < 0.05, One-Way ANOVA) (a); Relative mRNA expression levels of VEGF-A gene following Teucrium polium extract treatment. The expression of VEGF-A in the chick extra-embryonic membranes (n = 4 per experimental group) was decreased in the Teucrium polium treated group of 6 mg per kg egg-weight. The Teucrium polium extract was administered at dosages of 3 or 6 mg per kg egg weight (error bars show standard error of mean; *P < 0.05, OneWay ANOVA) (b).
4.3. VEGF-A Expression
The VEGF-A expression has been evaluated via the qPCR technique. The relative VEGF-A mRNA expression level decreased in the T. polium-treated group 3 compared to that in the control (Figure 4B).
5. Discussion
Herbal medicine is being used in various societies to treat different ailments. The drug toxicity is of great concern during pregnancy, and pregnant women should not use T. polium. Therefore, in this study, we assessed the embryonic vascular toxicity of T. polium via the EEM of chicks.
Our results suggest that vascular disorders and changes in the regular expression of specific genes associated with vasculogenesis can be generated by exposure to T. polium during the embryonic developmental period. These disorders are characterized as follows:
The first is the anti-angiogenic property and changes in the vascular branching pattern of the EEM, including reduced vessel area, vessel length vessels branching. It also increased in lacunarity. The highest disorder was severed in embryos treated with the herbal plant's high dose (6 mg/kg egg weight). Some studies have focused on the vascular lesions following herbal treatment (14, 15). The anti-angiogenic property of T. polium and the dosage in which the herb causes vascular defects in embryos are not clearly defined. Based on this study's results, T. polium causes vascular injury at dosages ≥ 6 mg/kg.
The vasculo-toxic effect of the T. polium is supported by some experimental data on the herb's biochemical content. According to several papers, the main activity of the T. polium has resulted from different substances that contain in the T. polium. The T. polium contains neoclerodane diterpenoids, monoterpenes, sesquiterpenes, polyphenols, flavonoids, and fatty acid esters (16, 17). Thus, alteration in vascular development may be due to these biochemical effects and may explain the decrease in vascular network proliferation in the vascular bed of T. polium-treated EEM.
Another explanation which accounts for the vascular injury due to T. polium administration is its cytotoxicity. It was showed that T. polium has cytotoxic effects on some established cell lines (18), and the cytotoxicity of T. polium has been demonstrated by other researchers (19).
The second T. polium disorder was reduced expression of VEGF-A. Alteration in gene expression and vascular development may link T. polium exposure and developmental defects of the fetus. T. polium was injected with doses of 3 or 6 mg/kg. The later dosage seems to have an adverse effect on gene expression. The results revealed the impact of T. polium on VEGF-A expression. We suggest the following mechanisms to explain the reduced expression of that gene. The decrease in vascular branching and angiogenesis induced by T. polium may have limited blood flow through the vessels. Changes in blood flow would reduce shear stress (20). VEGF-A is generally upregulated when shearing stress increases (21, 22). Therefore, the suggested reduction in shear stress after T. polium exposure may have decreased VEGF-A expression.
The in-silico analysis of the vessel plexus and MCA calculations on microscopic images used here have been widely applied in other vascular studies (23-25). The treatment time in the present study was chosen based on the previous experiment; vascular injuries were noted (8, 9, 26).
This study is the first to investigate the anti-angiogenic properties of T. polium via the EEM of a chick. It provides data on the vascular toxicity of T. polium to the fetus. Our findings overlap with previously reported data focusing on the vessel toxicity of the herbal plant. Additionally, the results show that T. polium alters genes' expression and induces adverse effects during vessel expansion. These injuries can cause pathological consequences on the human fetus that needs further investigation.
5.1. Conclusions
In conclusion, the CAM of chick is a valuable animal model for investigating the side effects of compounds, in which the experiment cannot be performed on the human fetus due to ethical aspects. It also helps physicians and clinicians for change in drug applications. The results reported here suggest that the use of the T. polium during gestation can have vascular toxicity, at least until further experiments are done on safety on the human embryo. We believe that the results achieved by this study will improve the clinician's knowledge about the adverse effects of this herb on the human fetus, particularly in industrial societies, because there is an increased reliance on the use of herbs. Therefore, T. polium consumption should be limited during pregnancy, and clinicians should determine the herb prescription during fetal growth or only be given when the benefit outweighs the risk.
Furthermore, T. polium applied to the chick EEM was vasculo-toxic at dosages ≥ 6 mg/kg weight. A lower dosage given during various stages of pregnancy caused far less pronounced vascular lesions. Therefore, the use of safe alternative herbs should be a high priority during fetal growth.