1. Context
Patients with urolithiasis constitute a great percentage of referrals to urology clinics. The prevalence of urolithiasis has been increasing recently (1). Urolithiasis is more common in men than in women (13% versus 7% in Western countries) (2). There is heightened awareness of renal stone disease in children, as well (3). In Iran, the prevalence of urolithiasis is 5.7 %, with an incidence of 145/100,000 (4). Metabolic diseases, urinary tract infections, and insufficient hydration could lead to recurrent urolithiasis. Calcium-containing stones are one of the most common stones that occur in 80% of the cases (5). The type of stone is affected by gender, age, and geographic location (6). Since plants are claimed to be safe, non-toxic, low-cost, and effective for the treatment of diseases, they are widely used by people (7). Phytotherapy helps in urolithiasis with several mechanisms including Litholytic, anti-inflammatory, diuretic, antimicrobial, and antispasmodic mechanisms (8).
Cystone® is a polyherbal tablet traditionally practiced in India and approved for the treatment of urolithiasis. Each tablet consists of Didymocarpus pedicellata (130 mg), Saxifraga ligulata (98 mg), Rubia cordifolia (32 mg), Cyperus scariosus (32 mg), Achyranthes aspera (32 mg), Onosma bracteatum (32 mg), and Vernonia cinerea (32 mg) and powders of Shilajeet (26 mg) and Hajrul yahood bhasma (32 mg). It has been approved by the Food and Drug Administration (FDA) after extensive studies on each ingredient (9). Cystone® prevents the super-saturation of calcugenicsubstances and controls oxamide. It prevents urolithiasis formation via the reduction of stone formers (10). By acting on the mucin, Cystone® leads to the disintegration of stones and crystals. Also, Cystone® has antimicrobial, antispasmodic, and anti-inflammatory properties that are helpful in the prevention of urinary tract infections associated with urolithiasis. Cystone® is known as Uricare® in the USA. It is manufactured worldwide by Himalaya Health Care (10).
The efficacy of Cystone® in urolithiasis has been studied frequently (9-13), but the results are inconsistent. Therefore, the impact of Cystone® as a natural remedy to keep stone formation and growth of existing stones was reviewed systematically.
2. Methods
2.1. Search Strategy
Published papers were searched for by two independent researchers in relevant databases including PubMed, Scopus, and Cochrane library up to December 30, 2017. Also, e-publications ahead of print were searched in PubMed. The quality of RCT studies was evaluated using the Jadad score (14).
2.2. Inclusion Criteria
The inclusion criteria were open-label or blinded-design Randomized Clinical Trials (RCTs), studying patients with nephrolithiasis. Full-text articles written in English, comparing Cystone®, with placebo or any active agent, aimed at dissolution or prevention (primary or secondary) of the formation of calculi in any part of the urinary tract were only included. The exclusion criteria were case-control studies, non-controlled studies, dose-finding studies, drug toxicity assessment, and animal or laboratory studies.
2.3. Outcomes
The primary outcomes were the decrease of stone number and size and the urinary excretion rate of calcium, urate, or oxalate. Any adverse effect of Cystone® was considered a secondary outcome.
2.4. Quantitative Data Synthesis and Data Analysis
The data were extracted and pooled with a meta-analysis approach. The results are expressed with 95% confidence intervals (95% CI). Heterogeneity was checked using the I2 statistics. Heterogeneity was considered significant if I2 was more than 50%. In a significant heterogeneity, data were analyzed using a random-effects model. P < 0.2 was considered as statistically significant. The data were analyzed using the comprehensive meta-analysis version 2.2.064 software.
3. Results
3.1. Search and Study Selection
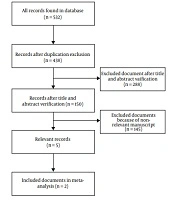
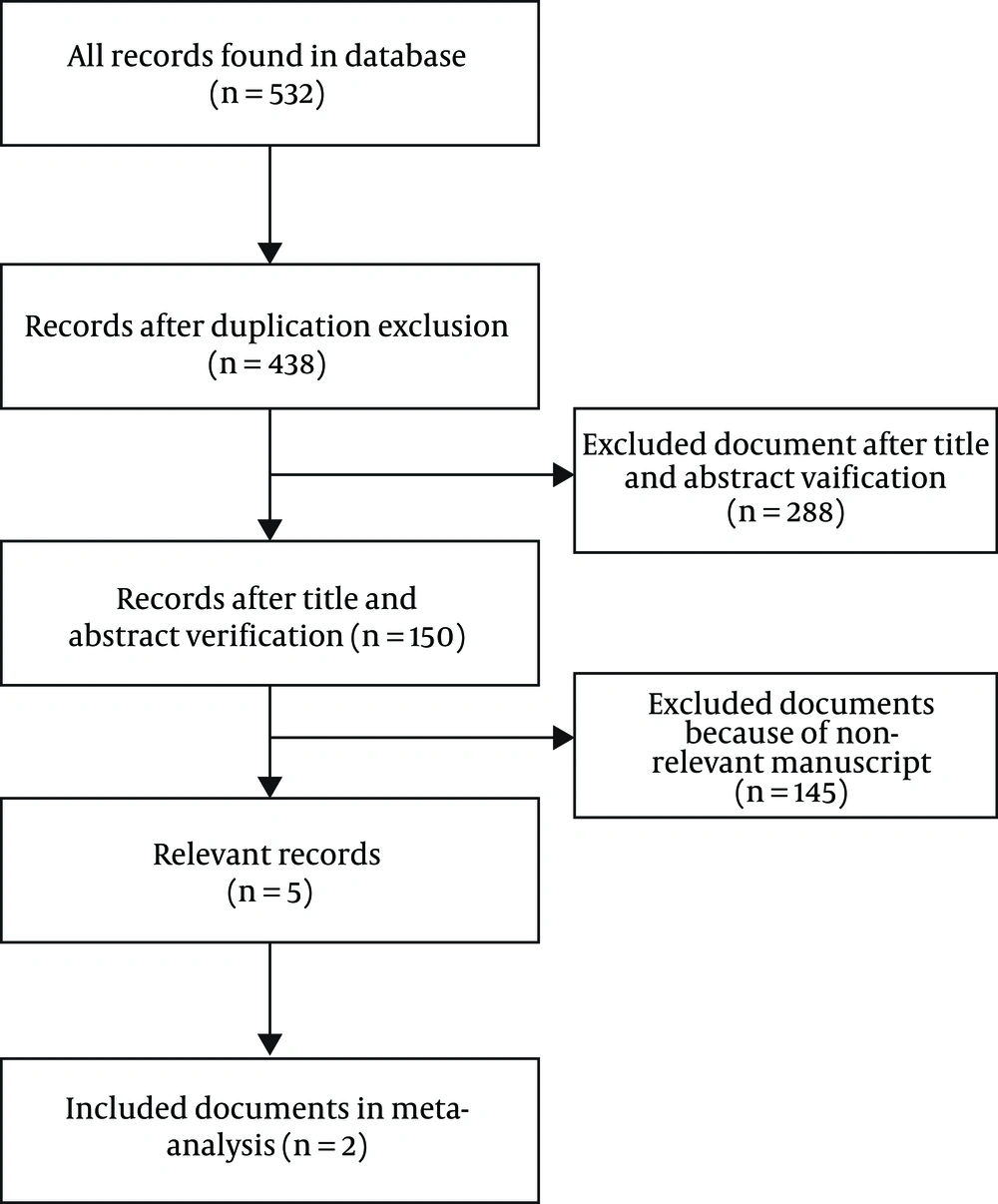
The literature search retrieved 532 relevant articles. Some articles were removed because of duplication. Some articles were excluded because they were books, book sections, review papers, or animal studies. Some studies were excluded because they had not a control group. Finally, five articles were included in the present review (9-12, 15). All were randomized clinical trials (RCTs). Among them, three studies had no interest data for the present systematic review (10, 11, 15). Therefore, they only were evaluated for the adverse effects of treatment. The diagram of the study selection process is presented in Figure 1. Details of the included studies are presented in Table 1.
| Name | Design | Sample Size (Control) | Sample Size (Intervention) | Control Group Regimen | Intervention Group Regimen | Follow-Up, mo |
|---|---|---|---|---|---|---|
| Erickson et al. (10, 11) | Crossover clinical study | 10 | 10 | placebo | Cystone | 3 |
| Stephen | Crossover clinical study | 10 | 10 | placebo | Cystone | 3 |
| Shekar Kumaran and Patki (12) | RCT | 30 | 30 | placebo | Cystone | 3 |
| Mohanty et al. (9) | RCT | 26 | 26 | placebo | Cystone | 6 |
| Palaniyamma | Before-after | - | 65 | - | Cystone | 3 |
3.2. Stone Size Change
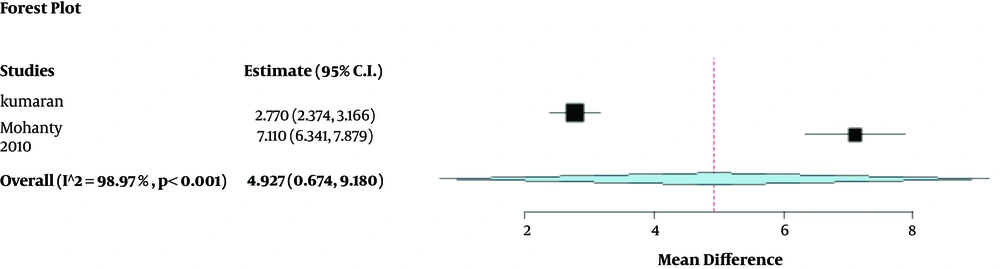
Changes in the calculi size were investigated in two studies (9, 12). In one study, Cystone® versus placebo was compared in two groups of patients according to the calculi size (5 to 12 mm). The mean size of stone decreased significantly in Cystone®-treated patients while the stone size increased in the placebo group (P < 0.0014). Also, 60% of the patients in the Cystone® group expelled stones completely, compared to 10% in the placebo group (P < 0.0006) (12). In another randomized study of 52 patients, Cystone® was compared with placebo in two groups of patients with the calculi size of 5 - 10 mm. The mean size of stone decreased in Cystone®-treated patients versus the placebo group (P < 0.001). Also, 50% of the patients in the Cystone® group expelled stones completely, compared to 7.69% in the placebo group (P < 0.0006) (9). The forest plots are shown in Figure 2. In a two-phase crossover study of 10 patients with calcium oxalate stones, Cystone® had not sufficient efficacy for the treatment of urolithiasis (10). In another two-phase crossover study of 10 patients with cystine stones, Cystone® had no significant effect on urolithiasis treatment in short term (six weeks) and long term (52 weeks) (11).
3.3. Urinary Excretion of Calcium and Uric Acid
Two studies included changes in urinary calcium and uric acid excretion rates (12, 15). In one study, only the final statistical difference between the Cystone® and placebo groups was shown and the mean or excretion rate was not reported. According to results, the difference between groups was significant in patients with calcium-oxalate stones (P < 0.05) but not other types of stones, including solitary and multiple stones (15). In the Shekar Kumaran and Patki study, the difference between the Cystone® and placebo groups was significant in the excretion rate of uric acid (mg/dL) (mean difference: -0.24 ± 0.17 in the Cystone® group versus 0.02 ± 0.8 in the placebo group but not in the excretion rate of calcium (mg/dL) (mean difference: -0.07 ± -0.22 in the Cystone group® versus -0.12 ± 0.3 in the placebo group (12).
3.4. Adverse Effects of Treatment
The side effects of treatment were reported only in one study (12), and in the other four studies, patients did not present any report about side effects (9-11, 15). Vomiting, gastric irritation, and dyspepsia in three patients in the Cystone® group versus dyspepsia in the placebo group were reported by Shekar Kumaran and Patki (12).
4. Discussion
The clinical data regarding the efficacy of Cystone® as an herbal remedy versus placebo in urolithiasis were systematically reviewed in the present study. Numerous studies have evaluated the efficacy of Cystone® clinically and experimentally, but only five studies met the inclusion criteria. Because of high heterogeneity (more than 90%), a random-effects model was used for the meta-analysis. Three studies had not eligible data for the meta-analysis. According to the meta-analysis, Cystone® had more efficacy for urolithiasis treatment than placebo. Cystone® made a significant difference in the decrease of stone size and expulsion rate compared to the placebo. Also, Cystone® almost had no adverse effect. The present study is the first systematical report evaluating the efficacy of Cystone® in urolithiasis treatment. However, most of the studies in phytotherapy had low quality. According to the search results, there were a few RCTs with standard protocols evaluating the efficacy of Cystone® versus placebo or any other active compound. We need more RCTs with stronger experimental designs, larger sample sizes with control groups, longer follow-ups, and more exact report of results.
According to the Shekar Kumaran and Patki study (12), there was no significant difference in the calcium rate between Cystone® and placebo. The effect of natural drugs, including Cystone® may be alter with the changes in risk factors. We propose that macromolecules in natural products could inhibit crystallization; they also contain digestive enzymes affecting the organic matrix of the stone.
Because of low costs and fewer adverse effects of phytotherapy in the treatment of urolithiasis, this approach is welcome for people. Herbal treatment is more welcome than synthetic drugs because patients suppose that natural products have low risks and side effects. Studies evaluating the effects of phytotherapy on urolithiasis are numerous but with low quality. A major problem in most studies was the lack of data about stone composition, which leads to problems in evaluating the effects of treatment. Also, ultrasound, as a more common imaging tool does not allow distinguishing between radiopaque and radiolucent calculus. Also, calcium calculus is solved slowly than uric acid calculus that is simply dissolved by alkalizing urine.
According to the literature, herbal products did not affect the main urinary risk factors. Therefore, patients should be aware of the low evidence of herbal treatment efficacy in urolithiasis. According to the present review, there is not enough evidence for a robust conclusion on the efficacy of Cystone® in urolithiasis treatment because of limited studies. Also, randomized clinical trials with strong designs are necessary to access strong evidence comparing the effect of Cystone® and other herbal treatments.
5. Conclusions
Cystone® has more efficiency in the treatment of urinary tract stones than has a placebo. It significantly induces stone size decrement and clearance compared to placebo. The low quality of reports is a major limitation in the applicability of these results.