1. Background
The pigment melanin is the main human skin substance that determines the color of human skin, hair, eyes, and a dark pigment produced by cells in the epidermis. Melanin protects skin cells against free radicals and UV irradiation (1). Freckles result from the increased activity of melanocytes and the accumulation of melanin in the skin (2). Tyrosinase is a metallo-enzyme responsible for the production of melanin in the skin. Tyrosinase inhibitors are used to treat hyper-pigmentary skin diseases and are usually present in skin whitening formulations (3). Tyrosinase is a key enzyme in the biosynthesis of melanin and is responsible for the darkening of the fruit and vegetables. Thus, compounds with tyrosinase inhibitory activities have dual roles and are used in the production of skin whitening cosmetics; also, pharmaceuticals are widely used in the food industry.
UV irradiation, stress, aging, and air pollution are inducers of hydrogen peroxide (H2O2) and other reactive oxygen species (ROS) in the melanogenesis process. The accumulation of free radicals in the skin leads to the destruction of cells, inflammation, a decrease in collagen synthesis, an increase in melanin synthesis, and DNA damage. They are ultimately manifested as wrinkles and hyperpigmentation (2). Antioxidants in the body are among the most significant defense mechanisms that reduce and neutralize free radicals to delay aging and prevent skin damage (4).
Pistacia, a genus of flowering plants belonging to the Anacardiaceae family, grows in different regions in Iran. Pistacia atlantica subsp. Kurdica is a tree over 25 m in height, and its local name is “Baneh”. Different parts of the plant are used in folk medicine for various treatment conditions, such as eczema, kidney stones, throat infections, upper abdominal discomfort and pain, peptic ulcer, oral diseases, dyspepsia, diarrhea, and asthma (5, 6). Several biological properties, such as anticancer, antioxidant, antipyretic, antimicrobial, antibacterial, anti-inflammatory, antiviral, anti-diabetic, anti-radical, and cytotoxic activities can be partly attributed to phenolic compounds present in the plant (7-9). However, there are no scientific reports on the anti-melanogenesis inhibitory activity of P. atlantica subsp. Kurdica.
2. Objectives
This study aimed to investigate the inhibitory effect of methanol (MeOH), n-hexane, dichloromethane (CH2Cl2), n-butanol (BuOH), ethyl acetate (EtOAc), H2O extracts, and P. atlantica subsp. Kurdica essential oil on the melanogenesis process and to evaluate the potential antioxidant activity of the plant on B16F10 melanoma cells.
3. Methods
3.1. Preparation of Extracts
Unripe P. atlantica subsp. Kurdica fruits were collected in May 2014 from Kermanshah, West Iran. Voucher specimens were identified in the herbarium of the Faculty of Pharmacy, the Mashhad University of Medical Sciences, Mashhad, Iran. Unripe P. atlantica subsp. Kurdica fruits (225 g) were extracted with pure methanol for 24 h using the percolation method at room temperature according to the previously reported protocol. The whole methanol extract was filtered, and the solvent was evaporated using a rotary evaporator and then freeze dried to afford a crude methanol extract. This extract (100 g) was then suspended in 95% methanol and successively partitioned between n-hexane, dichloromethane, ethyl acetate, n-butanol, and water.
3.2. Isolation of the Essential Oil
The essential oil from the unripe P. atlantica subsp. Kurdica fruits (150 g) was obtained through wet steam distillation using a clevenger-type apparatus.
3.3. GC and GC-MS Analysis
The GC analysis was performed using a Varian CP-3800 equipped with an FID detector, interfaced with a fused-silica column (CP-Sil 8CB, 50 m × 0.25 mm, film thickness 0.12 m), at an oven temperature of 50°C - 250°C with the rate of 3°C/min, injector temperature of 260°C, the split ratio of 1:5 with carrier gas, the N2 flow rate of 2 mL/min, and detector temperature of 280°C (10).
3.4. Cell Culture
B16F10 (melanoma cell line, Cat. No.: C540) was purchased from the Pasteur Institute (Tehran, Iran). The extract activity in each experiment was calculated using the following formula: % of activity = (Absorbance of the sample × 100)/Absorbance of the control.
3.5. Cell Viability Assay
Resazurin is a non-toxic, non-fluorescent, and cell-permeable cell health indicator that converts to a highly fluorescent and red compound, called resorufin, after reducing cytosol of live cells (10, 11).
3.6. Mushroom Tyrosinase Activity Assay
Mushroom tyrosinase activity was measured by modifying the method described by Kim et al. previously (12).
3.7. Determination of the Melanin Content
The melanin content was measured, as described previously (13).
3.8. Cellular Tyrosinase Activity Assay
Tyrosinase activity was determined by spectrophotometry following the oxidation of DOPA to DOPAchrome (10).
3.9. Determining the Level of Cellular ROS
The reactive oxygen species level with minor modifications was measured, as described previously (10).
3.10. Western Blotting Analysis
B16F10 melanoma cells were cultured in 75 cm2 flasks, as described previously (10).
3.11. Statistical Analysis
All experiments were repeated in triplicate, and the relative results were presented as the mean ± SD of the three different measurements. Variance analysis was performed using a one-way ANOVA test with GraphPad Prism 6.0 to examine quantitative differences between the groups. The means were compared by Dunnett tests. P < 0.05 was considered statistically significant.
4. Results
4.1. Essential Oil Composition
The 65 components were identified as volatiles, representing 99.8% of the essential oil composition after the GC-MS analysis of oil obtained from unripe P. atlantica subsp. Kurdica fruits through hydro-distillation (Table 1). The grouped contents of the essential oil were determined as monoterpene hydrocarbons 85.8%, oxygenated monoterpenes 6.3%, sesquiterpene hydrocarbons 5.7%, oxygenated sesquiterpens 1.6%, and the miscellaneous 0.6%. The major oil components of the essential oil were α-pinene 60.1%, myrcene 8.0%, and β-pinene 5.2%.
| Number | Compound | KIa | Percentage |
|---|---|---|---|
| 1 | α-pinene | 940 | 60.1 |
| 2 | Camphene | 949 | 2.8 |
| 3 | Verbenene | 962 | 0.3 |
| 4 | Benzaldehyde | 960 | tb |
| 5 | β-pinene | 978 | 5.2 |
| 6 | Myrcene | 986 | 8.0 |
| 7 | α-phellandrene | 1000 | 0.4 |
| 8 | δ-3-carene | 1018 | 0.1 |
| 9 | α-terpinene | 1015 | 0.2 |
| 10 | ρ-cymene | 1023 | 0.2 |
| 11 | Limonene | 1029 | 2.8 |
| 12 | Z-β-ocimene | 1033 | 3.1 |
| 13 | E-β-ocimene | 1052 | 0.8 |
| 14 | γ-terpinene | 1059 | 0.2 |
| 15 | Terpinolene | 1082 | 1.6 |
| 18 | 6-camphenol | 1095 | t |
| 17 | Perillene | 1087 | t |
| 16 | n-nonanal | 1090 | t |
| 19 | Exo-fenchol | 1115 | 0.2 |
| 20 | α-campholenal | 1126 | 0.3 |
| 21 | Allo-ocimene | 1130 | t |
| 22 | Trans-pinocarveol | 1139 | 0.2 |
| 23 | Camphor | 1141 | t |
| 24 | Camphene hydrate | 1148 | 0.1 |
| 25 | Isoborneol | 1156 | t |
| 26 | Trans-pinocamphone | 1160 | t |
| 27 | Pinocarvone | 1164 | 0.1 |
| 18 | Borneol | 1167 | 0.3 |
| 29 | ρ-mentha- 1,5-8-ol | 1170 | 0.3 |
| 30 | Terpinen-4-ol | 1176 | 0.2 |
| 31 | ρ-methyl acetophenone | 1184 | t |
| 32 | α-terpineol | 1190 | 2.7 |
| 33 | Myrtenol | 1195 | 0.1 |
| 34 | Verbenone | 1205 | 0.1 |
| 35 | Trans-carveol | 1216 | t |
| 36 | Isobornyl acetate | 1285 | 1.2 |
| 37 | Trans- pinocarvyl acetate | 1298 | t |
| 38 | 3E-hexenyl tiglate | 1317 | t |
| 39 | α-terpenyl acetate | 1349 | 0.1 |
| 40 | α-copaene | 1373 | t |
| 41 | 3Z-hexenyl hexanoate | 1385 | t |
| 42 | Iso-caryophyllene | 1408 | t |
| 43 | β-caryphyllene | 1419 | 4.3 |
| 44 | Aromadendrene | 1436 | t |
| 45 | α-humulene | 1450 | 0.9 |
| 46 | α-amorphene | 1472 | t |
| 47 | Germacrene D | 1479 | 0.1 |
| 48 | Bisyclogermacrene | 1495 | 0.1 |
| 49 | α-muurolene | 1500 | t |
| 50 | δ-amorphene | 1512 | 0.1 |
| 51 | 3z-hexenyl benzoate | 1566 | 0.3 |
| 52 | Spathulenol | 1575 | 0.1 |
| 53 | n-hexyl benzoate | 1576 | 0.1 |
| 54 | Caryophyllene oxide | 1580 | 0.1 |
| 55 | 2-e-Hexenyl benzoate | 1583 | 0.1 |
| 56 | Guaiol | 1596 | t |
| 57 | Eremoligenol | 1625 | t |
| 58 | γ-eudesmol | 1629 | 0.3 |
| 59 | β-caryophylla-4(12)-8(13)-dien-5-ol | 1632 | t |
| 60 | β-eudesmol | 1645 | 0.4 |
| 61 | α-eudesmol | 1650 | 0.5 |
| 62 | 7-epi-α-eudesmol | 1658 | t |
| 63 | Unknown | 1666 | t |
| 64 | Unknown | 1742 | t |
| 65 | Methyl hexadecanoate | 1921 | t |
| Major Grouped Compounds | |||
| Monoterpene hydrocarbons | 85.8 | ||
| Oxygenated monoterpenes | 6.3 | ||
| Sesquiterpene hydrocarbons | 5.7 | ||
| Oxygenated sesquiterpenes | 1.6 | ||
| Miscellaneous compounds | 0.6 | ||
Volatile Components of the Hexane Extract Obtained from Oleoresins of P. atlantica Subsp. Kurdicaa
4.2. Effect of Pistacia atlantica Subsp. Kurdica Fractions and Essential Oil on Cell Survival
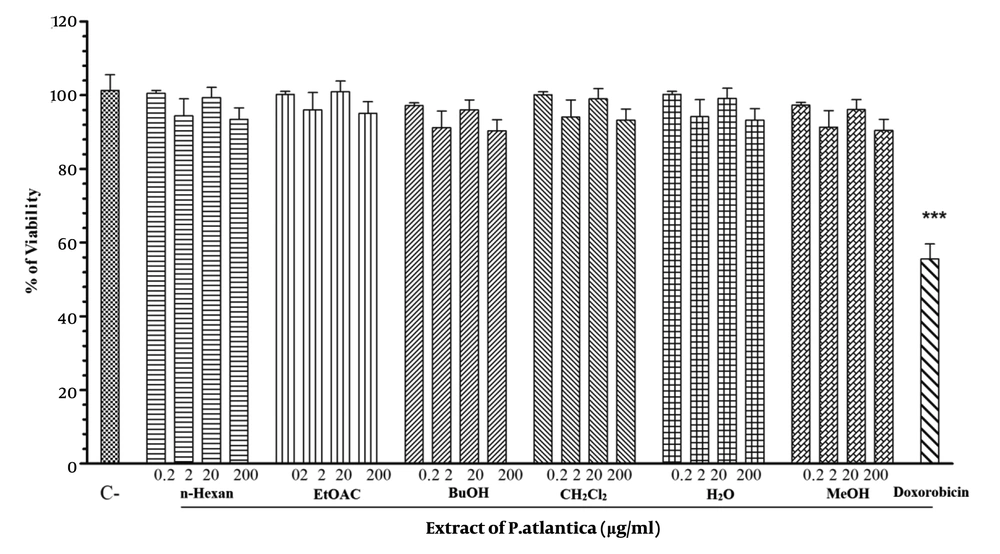
Resazurin assay was performed to monitor cell viability. B16F10 cell lines were seeded in a 96-well plate. After 24 h, cells were treated with different concentrations (0.2 - 200 µg/mL) of P. atlantica subsp. Kurdica fractions and essential oil. The results showed that the fractions had no significant cytotoxic effect on B16F10 cells at the above concentrations, although the 200 µg/mL concentration of the essential oil had a cytotoxic effect. Doxorobicin as a positive control significantly induced cell death (P < 0.01) (Figure 1).
4.3. Effect of Pistacia atlantica Subsp. Kurdica Fractions and Essential Oil on Melanin Synthesis
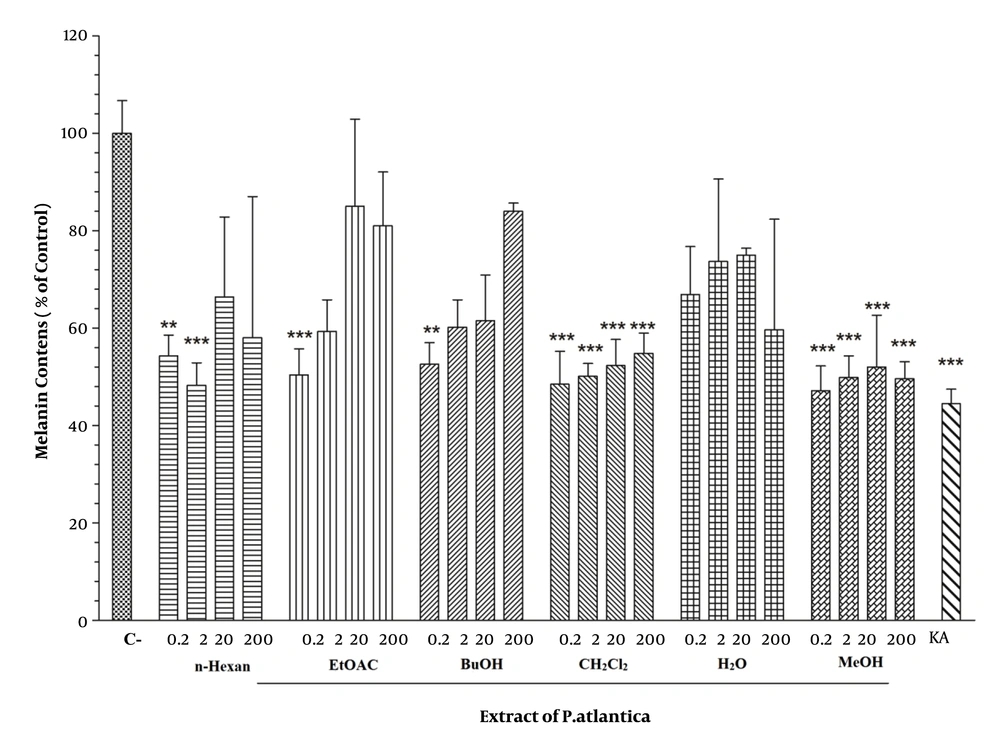
The melanin content of fraction-treated B16F10 melanoma cells was quantified to study the effect of different concentrations of P. atlantica subsp. Kurdica fractions and essential oil on melanin synthesis. Kojic acid was utilized as a positive standard. All fractions significantly decreased the melanin content in the cells. The 0.2, 2, 20, and 200 µg/mL concentrations of MeOH and the 0.2, 2, 20, and 200 µg/mL concentrations of the CH2Cl2 fraction had inhibitory effects on melanin synthesis. However, the 0.2 µg/mL concentration of EtOAC and the 2 µg/mL concentration of n-hexane fraction had a significant inhibitory effect on melanin synthesis. Also, none of the essential oil concentrations had inhibitory effects. However, Kojic acid as a positive control significantly decreased the melanin content (Figure 2).
4.4. Effect of Pistacia atlantica Subsp. Kurdica Fractions and Essential Oil on Mushroom Tyrosinase Activity
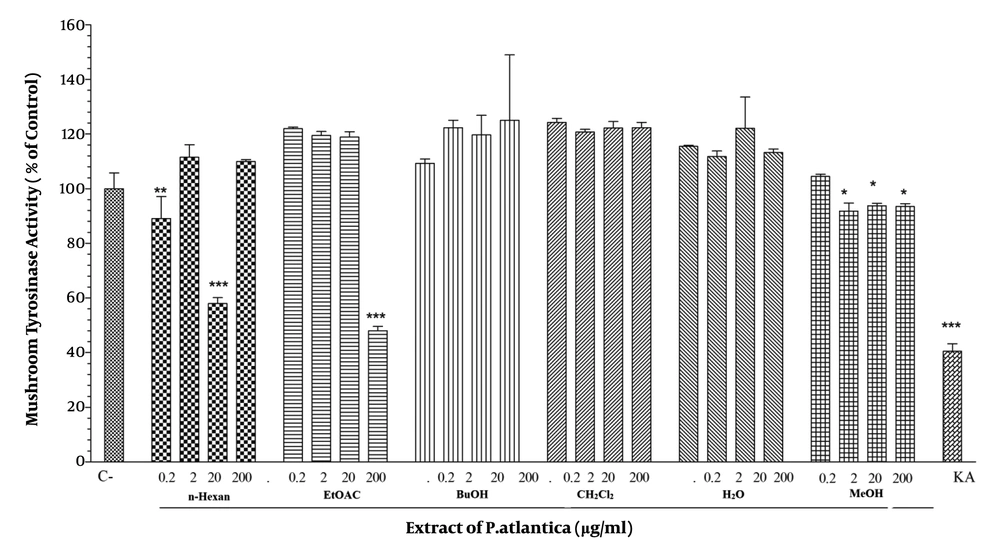
We performed a mushroom tyrosinase assay using L-DOPA as the substrate and mushroom tyrosinase as the enzyme source to determine whether P. atlantica subsp. Kurdica extracts and essential oil would affect tyrosinase activity directly. The results showed the 20 µg/mL concentration of n-hexane fraction and the 200 µg/mL concentration of EtOAC exerted a significant inhibitory effect on L-DOPA oxidation by mushroom tyrosinase and significantly reduced the mushroom tyrosinase activity. However, none of the essential oil concentrations had inhibitory effects (Figure 3).
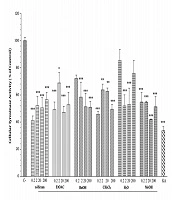
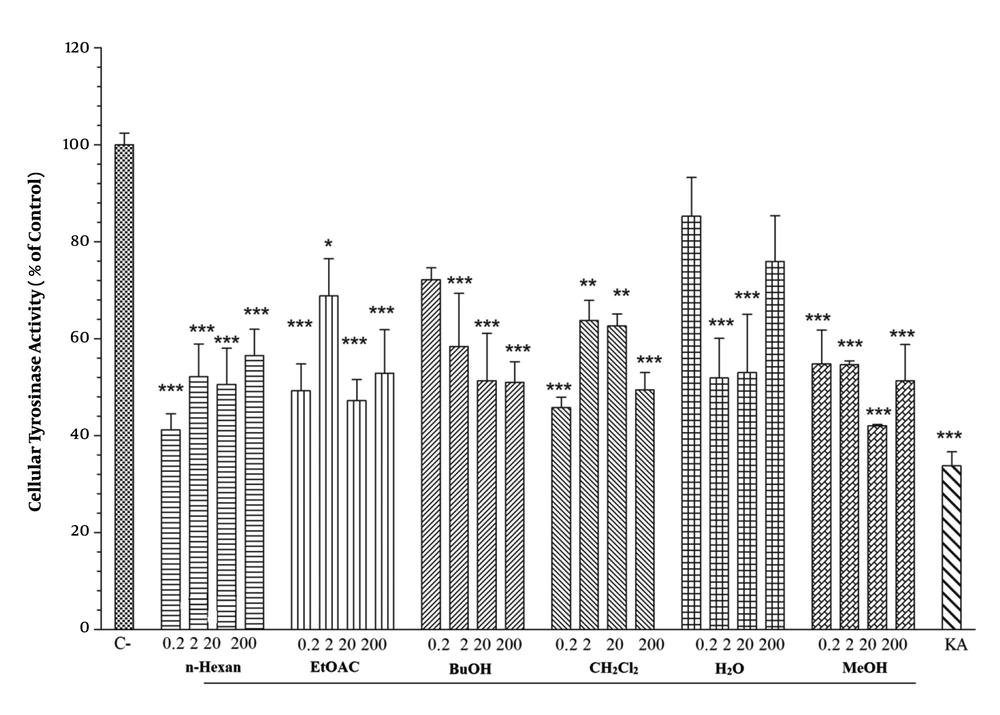
4.5. Effect of Pistacia atlantica Subsp. Kurdica Fractions and Essential Oil on Cellular Tyrosinase Activity
We assessed the anti-melanogenesis effect of P. atlantica subsp. Kurdica fractions and essential oil on cellular tyrosinase activity. The results indicated that the 20 µg/mL concentration of MeOH and the 0.2 µg/mL concentration of n-hexane fractions had inhibitory effects on the cellular tyrosinase activity. The BuOH and CH2Cl2 fractions of P. atlantica subsp. Kurdica at 0.2 µg/mL and EtOAC at 20 µg/mL significantly inhibited the cellular tyrosinase activity. However, different essential oil concentrations showed no inhibitory effects (Figure 4).
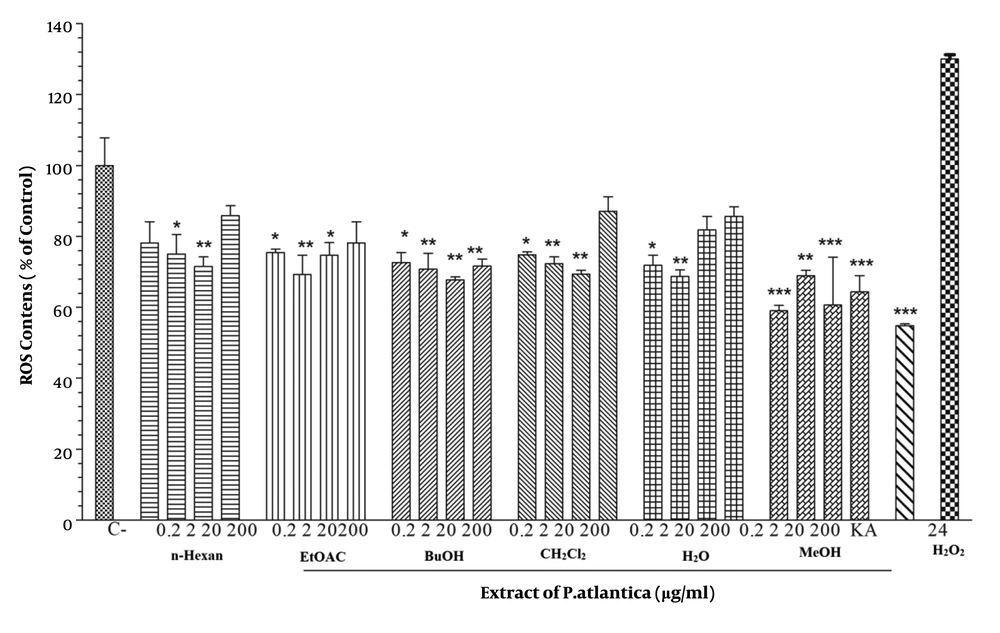
4.6. Effect of Pistacia atlantica Subsp. Kurdica Fractions and Essential Oil on Cellular Reactive Oxygen Species Level
The antioxidant capacity of different fractions was measured regarding intracellular ROS levels. An amount of 24 mM of H2O2 was exposed to the cells pretreated with different P. atlantica subsp. Kurdica fractions and essential oil concentrations. The results indicated that although all concentrations of MeOH fractions of P. atlantica subsp. Kurdica significantly suppressed oxidative stress induced by H2O2, none of the essential oil concentrations suppressed oxidative stress (Figure 5).
4.7. The Level of Tyrosinase and MITF Protein Pistacia atlantica Subsp. Kurdica Fractions
We performed western blot analysis to determine the tyrosinase protein level in cells treated with different P. atlantica subsp. Kurdica fractions. The results indicated that tyrosinase protein levels significantly decreased by the EtOAC extract of P. atlantica subsp. Kurdica at 200 µg/mL. These results suggest that the anti-melanogenesis effect of the mentioned fractions on B16F10 cells is associated with down regulating tyrosinase protein expression and inhibiting melanin production. β-Actin was used as an internal control.
5. Discussion
Chemicals and natural base preparations are widely used to prevent skin hyper pigmentation disorders. A major factor determining skin color is melanin that prevents UV induced skin damage. The melanin content change in the skin is correlated with conditions such as leukoplakia, albinism, freckles, melasma, moles, and lentigo. The main treatment strategy for over-pigmentation diseases is the application of skin whitening agents. Arbutin, hydroquinone, and kojic acid are among the numerous compounds used for pigmented skin (14).
In melanocytes, melanin is synthesized by the enzymatic interaction of tyrosinase, tyrosinase-related protein 1 (TRP1), and tyrosinase-related protein 2 (TRP2). A transformation of tyrosine into 3,4-dihydroxyphenylalanine (L-DOPA) and a conversion of L-DOPA into DOPA quinine are the first steps of melanogenesis (15).
In this study, different P. atlantica subsp. Kurdica extracts (MeOH, CH2Cl2, EtOAC (0.2 µg/mL), and n-hexane (2 µg/mL)) significantly decreased melanin synthesis without showing cytotoxicity. It was found that all the extracts had a tyrosinase inhibitory effect on B16F10 melanoma cells.
There exist a number of mechanisms to regulate melanogenesis (16). Tyrosinase is the rate limiting enzyme of melanogenesis and the most common target for depigmenting agents. Ascorbic acid, phenolic compounds, thio-containing compounds, and kojic acid used for whitening ingredients inhibit tyrosinase enzyme activity (1).
In this study, we observed that the P. atlantica subsp. Kurdica extracts reduced tyrosinase activity by affecting cellular tyrosinase in B16F10 cells. These results indicate that the anti-melanogenesis activity of P. atlantica subsp. Kurdica is included in superior levels regulating tyrosinase enzyme such as maturation, translation, and transcription (17). We determined whether the P. atlantica subsp. Kurdica extracts could inhibit tyrosinase activity to investigate the anti-melanogenesis activity mechanism of P. atlantica subsp. Kurdica. In this study, we used mushroom tyrosinase as an enzyme source and kojic acid as a positive control. The results showed that the n-hexane (20 µg/mL) and EtOAC (200 µg/mL) extracts of P. atlantica subsp. Kurdica significantly decreased mushroom tyrosinase activity after 24 h of treatment.
Antioxidants have been widely used to prevent and treat disorders related to oxidative stress in dermatological disorders such as wrinkle forming and aging, both experimentally, or in cosmetic industry. It has been reported that free radicals and reactive oxygen species (ROS) cause skin inflammation and ageing (18, 19). ROS and UV-irradiation stress plays a major role in photo-aging (10, 20). Moreover, antioxidants play an essential role in reducing oxidative stress or damage in the human body, and flavonoids and phenolics have shown effectiveness in reducing melanogenesis (21, 22).
Many reports confirm that plants have antioxidant activities and decrease oxidative stress, and may have a high potential for skin disorder treatment. For example, Gourine et al. (23) showed that the P. atlantica aerial parts had powerful antioxidant properties. In another research, different extracts of the P. atlantica aerial parts reduced oxidative stress, which may be attributed to polyphenols, flavonoids, and anthocyanin widely found in the plant (5, 24). Generally, herbal antioxidants reduce oxidative stress in cells and have protective effects against oxidative stress-related disorders (25).
In this study, we examined the antioxidant activity of the P. atlantica subsp. Kurdica extracts. The result showed that all the concentrations of the MeOH fractions of P. atlantica subsp. Kurdica could significantly decrease oxidative stress induced by H2O2. The anti-melanogenesis activity of P. atlantica subsp. Kurdica might be due to antioxidant activity. Flavonoids are major active compounds in P. atlantica subsp. Kurdica with potent antioxidant agents (26).
Taken together, a decrease in ROS production, the tyrosinase protein level, the amount of melanin in cells, and the mushroom tyrosinase activity indicate the inhibition of melanogenesis in B16F10 cells by P. atlantica subsp. Kurdica. Based on our results, anti-tyrosinase and anti-melanogenic effects are due to the presence of polar compounds, and not non-polar and volatile compounds, in the extract. Hence, P. atlantica subsp. Kurdica with dual anti-melanogenic and antioxidant actions may contribute to skin whitening compounds and could be included in cosmetic formulations of skin care products.
5.1. Conclusions
This is the first report regarding the effect of P. atlantica subsp. Kurdica on melanin production. The present study’s results revealed that P. atlantica subsp. Kurdica extracts significantly inhibited tyrosinase activity and decreased melanin synthesis. Moreover, P. atlantica subsp. Kurdica exhibited intracellular free radical scavenging activity. It can be concluded that the P. atlantica subsp. Kurdica extracts are effective inhibitors of melanogenesis and can be useful as a therapeutic treatment for skin hyperpigmentation disorders.