1. Background
Teucrium polium (Lamiaceae family), known popularly as poly or germander, is named “Kalpooreh” in Persian. It’s a wild-growing plant native to the north, west, and south of Iran. Its medicinal use dates back to the time of Galen and Hippocrates, mainly to treat headache, convulsion, gastrointestinal disorders, and dysmenorrhea (1). Several studies have mentioned to hypoglycemic, hypolipidemic, anti-inflammatory, antioxidant, and other medicinal properties of the polium (2). Meanwhile, there are studies that mentioned to anticancer effects of this plant on some cancerous cell lines (2).
However, Khader et al. (3) reported no antimutagenic or cytoprotective activity of aqueous extract of T. polium. While Khader et al. (4) showed that the ethanol extract of T. polium had an inhibitory effect on N-methyl-N’-nitro-N-nitrosoguanidine mutagenicity.
According to the study by Nematollahi-Mahani et al. (5), the ethanolic extract of T. polium affects the proliferation of cell lines of BT20, MCF-7, A549, and PC12 in the adrenal medulla of rats.
Ljubuncic et al. (6) found that the water extract of T. polium had an antioxidant effect, but they could not show the cytotoxicity effect of the extract in PC12 and HepG2 (human liver carcinoma) cell lines.
In a study conducted by Rajabalian (7), the combined use of methanol extract of T. polium (Me-TP), doxorubicin, vincristine, and vinblastine was more effective in inhibiting the growth of Skmel-3 (melanoma), Saos-2 (osteoblastoma), MCF-7 (breast carcinoma), SW480 (colon carcinoma), EJ (bladder carcinoma, KB (oral cavity epidermal), and A431 (epidermoid carcinoma) cell lines than sole administration of each.
Menichini et al. (8) showed the cytotoxic effects of the essential oils of T. polium on COR-L23, CACO-2), and C32 cell lines.
The cytotoxic effect of aqueous and methanol extracts of T. polium on REYF-1 cell line is evaluated by Eskandary et al. (9).
Kandouz et al. (10) indicated the inhibitory effect of T. polium aqueous extract on cell proliferation of DU145 and PC3 human prostate cancer cell lines.
Due to increased life expectancy and increased exposure to various risk factors, the incidence of cancer has increased in Iran and is expected to continue increasing during the next two decades.
Due to increased life expectancy and increased exposure to various risk factors, the incidence of cancer has increased in Iran (11) and is expected to continue increasing during the next two decades (12). A study conducted in 2006 reported an incidence rate of 7 - 8 per 100,000 for males and females in Iran (13).
2. Objectives
Regarding the high prevalence of cancer (all types) in Iran and the cytotoxic properties of T. polium, further evaluation of the preventive and therapeutic effects of this endemic plant on other types of cancer cell lines seems to be rational. In this line, the current study aimed to investigate the cytotoxic and anti-mutagenicity effects of the essential oil of T. polium on HT29 (human colon adenocarcinoma) cell line.
3. Methods
3.1. Preparation of Essential Oil
Fresh aerial parts of T. polium were collected in October 2015 from Genow protected area (Hormozgan Province, Iran). Specimens were identified by Dr. G. Amin (School of Pharmacy, Tehran University, Tehran, Iran) and vouchers were deposited in the herbarium of the school of Pharmacy under the code number of PMP: 364.
We used the Clevenger-type apparatus to hydro-distillate the aerial parts. After 3 hours, the oil was collected and dried with anhydrous Na2SO4. Finally, the oil was measured and transferred into the clean glass vials for further investigations.
3.2. GC Analysis of the Essential Oil
Analyses were performed on a GC apparatus (Model Hp-6890) equipped with an FID and DB-5 column (0.25 mm × 30 m, 0.25 µm film thickness), and the column was raised from 60°C to 240°C at a rate of 4°C /min. N2 was used as a carrier gas at a flow rate of 2.0 mL/min injector port. The temperatures of the detector were 250°C and 300°C, respectively.
Gas chromatography/mass spectrometry (GC/MS) analysis was performed on a GC apparatus (Model HP6890, Hewlett Packard) coupled with an MS apparatus (model HP5972, Hewlett Packard) with a DB-5 capillary column (30 m × 0.25 mm; 0.25 µm film thickness). The carrier gas was helium, and the other operating conditions were the same as described above. Mass spectra were acquired at 70 EV. The mass scan range was 40 - 400 m/z at a sampling rate of 1.0 scan/s. The electronic integration of the FID peak areas was used for the quantitative determination of the compound. To identify the components of the oil, their retention time, retention indices, relative to C9-C28 n-alkanes, and computer matching with the Wiley 275.L library were performed, and the pattern of MS fragmentation and relative retention time were compared with the reference compounds in the literature (14, 15).
The percentage of the composition was then calculated based on the GC peak areas without any correction factors.
3.3. Cell Culture
Human colon adenocarcinoma cell line HT29 was purchased from the Pasteur Institute, Tehran (Iran), and was cultivated in T75 tissue culture flasks in RPMI-1640 supplemented with 10% fetal bovine serum. Then, the cells were incubated in a humidified incubator containing 5% CO2 at 37°C. After the growth and reproduction of the cells, adherent cells were separated from the flask bottom using the trypsin 0.25%. Afterward, 5000 cells were counted and transferred to flasks. After 18 hours of incubation in flasks of cell culture plates, the analyses were performed.
3.4. Microculture Tetrazolium Technique Assay
To determine the viability of cells under different conditions, the microculture tetrazolium technique (MTT) assay was used (16, 17). Since MTT is capable of reducing the MTT, it’s based on the reducing MTT to purple Formazan by mitochondrial reductase in alive cells.
After 18 hours, various concentrations of the essential oil of T. polium (TpEO) were added to cells, and the plates were incubated at 37°C and 5% CO2 for 48 hours. 96-well plate was performed for seeding HT29 cells (5 - 7 × 103 cells per well). TpEO in different concentrations (0, 10, 25, 50, 75, 100, 150 µg/mL) was added to each well and incubated. After 48 hours, incubation with 5 µg/mL MTT for 4 h was done.
After centrifugation, the supernatant was removed, and, eventually, 100 µL of Dimethyl sulfoxide (DMSO) was added to each well. For the MTT assay, we used forty-eight wells. ELISA reader was used to measure the absorbance of the cells at 570 nm.
The toxicity level was calculated by the following formula (17):
Viability % = 100 - cytotoxicity %
To diminish the test error, two measures were performed: (A) MTT strain was added to some wells without cells, and (B) the absorbance level was subtracted from whole the absorbance.
3.5. IC50 (Inhibition Concentration) Determination
Determining the 50% inhibition concentration (IC50) value for the TpEO on HT29 cells after 48h was performed by the probit analysis, using the Pharm Pharmacologic Calculation System (PCS) statistical package (Springer- Verlag, USA).
3.6. Sub-G1 Apoptosis Flow Cytometric Analysis
To analyze the cells for sub-G1 apoptosis, we followed the methodology used by Karimi et al. (16). Flow cytometric analysis of PI-labeled cells was used to determine the cellular DNA content.
Cells were seeded at a density of 106 cells per well into a 24-well plate and grown to exponential phase, and then were incubated for 24h and again for 48h after treating with the 50% inhibition concentration.
Cells were harvested, fixed in ice-cold 70% ethanol at 4°C, washed with PBS (phosphate-buffered saline) (PH = 7.2), treated with 25 µg/mL RNase at 37°C for 15 min, and stained with 50 µg/mL propidium iodide (PI) for 20 min. After incubation, a FACS caliber apparatus was used to measure the PI fluorescence of individual nuclei (18).
3.7. Apoptotic Changes in the Plasma Membrane
Apoptosis was detected by staining the cells with Annexin V using an Annexin-V-FLUOS Staining Kit (Roch), approximately 106 cells (as determined using a hemocytometer) were analyzed according to the protocol provided by the manufacturer.
In summary, cells were resuspended in Annexin-V-FLUOS labeling solution after being washed once with the phosphate-buffered solution.
The cells were incubated at 15°C - 25°C for 10 - 15 min. A flow cytometer (Becton- Dickinson) was used to measure the samples (19).
3.8. Antimutagenesis Test
For the Ames test, the fresh bacterial culture of Salmonella typhimurium TA100 was used.
Appropriate bacterial concentration was considered 1 - 2 × 109 cells/ml. The 50% inhibition concentration (IC50 ) values of the TpEO were added to the test tube containing 10 mL top agar (50 g/L Agar + 50 g/L NaCl), 0.5 mL of the overnight fresh bacterial culture, sodium azide (as a carcinogen) (1.5 µg/mL Sodium azide), 0.5ml of histidine, and biotin solution (0.5 mM histidin/0.5 mM biotin). Then, the content of the tube was distributed on the surface of the medium of glucose agar (40% glucose). The incubation was performed at 37°C after 3 seconds shaking 48 hours.
Each treatment was repeated 3 times, the reversed colonies were counted after 48 h incubation at 37°C. Antioxidant activity (prevention percentage) was calculated as follows (20):
T is reversed colonies in each Petri dish under carcinogen + TpEO, and M is reversed colonies in Petri dishes related to the positive control (mutagen).
3.9. Statistical Analysis
The data were analyzed using SPSS version 17. The results are expressed as the mean ± SD of the indicated number of independent experiments. One way analysis of variance (ANOVA) with Tukey’s test was used to compare the means of obtained values. A P value of < 0.05 was considered as statistically significant.
4. Results
A yellow oil with a yield of 0.2 % (v/w) was obtained from hydro-distillation of T. polium.
As shown in Table 1, nearly 81.1 % of its total composition are presented by twenty-six identified in oil components. The main constituent of the oil were β-caryophyllene (3.1 %), α-pinene (4.5 %), elemol (7.1 %), spathulenol (8.2 %), and 10-epi-γ-eudesmol (41.7 %).
| Compounda | KIb | KIc | Percentage | |
|---|---|---|---|---|
| 1 | α-Pinene | 935 | 939 | 4.5 |
| 2 | β-Pinene | 973 | 980 | 2.1 |
| 3 | β-Myrcene | 994 | 991 | 1.5 |
| 4 | Limonene | 1027 | 1031 | 1.7 |
| 5 | trans-β-Ocimene | 1045 | 1050 | 0.1 |
| 6 | Campholenal | 1119 | 1125 | 0.3 |
| 7 | cis-Sabinol | 1142 | 1140 | 0.6 |
| 8 | trans-3-Carene-2-ol | 1191 | 1192 | 1.8 |
| 9 | Myrtenal | 1201 | 1193 | 0.8 |
| 10 | Verbenone | 1209 | 1204 | 1.1 |
| 11 | Carvone | 1239 | 1242 | 0.2 |
| 12 | Eugenol | 1352 | 1356 | 0.1 |
| 13 | β-Bourbonene | 1381 | 1384 | 0.2 |
| 14 | β-Elemene | 1389 | 1391 | 0.2 |
| 15 | β-Caryophyllene | 1422 | 1418 | 3.1 |
| 16 | α-Humulene | 1449 | 1455 | 0.4 |
| 17 | Germacrene D | 1476 | 1480 | 1.2 |
| 18 | β-Selinene | 1487 | 1485 | 0.8 |
| 19 | β-Guaiene | 1489 | 1490 | 0.1 |
| 20 | Valencene | 1495 | 1491 | 1.4 |
| 21 | α-Panasinsene | 1522 | 1518 | 1.2 |
| 22 | Elemol | 1551 | 1547 | 7.1 |
| 23 | Spathulenol | 1577 | 1576 | 8.2 |
| 24 | Caryophyllene oxide | 1587 | 1581 | 0.3 |
| 25 | 10-epi-γ-Eudesmol | 1598 | 1602 | 41.7 |
| 26 | Ledene oxide | 1642 | 1646 | 0.4 |
| Total | 81.1 |
GC-MS Analysis of the Essential Oil From T. polium Aerial Parts
4.1. Vital Capacity Test
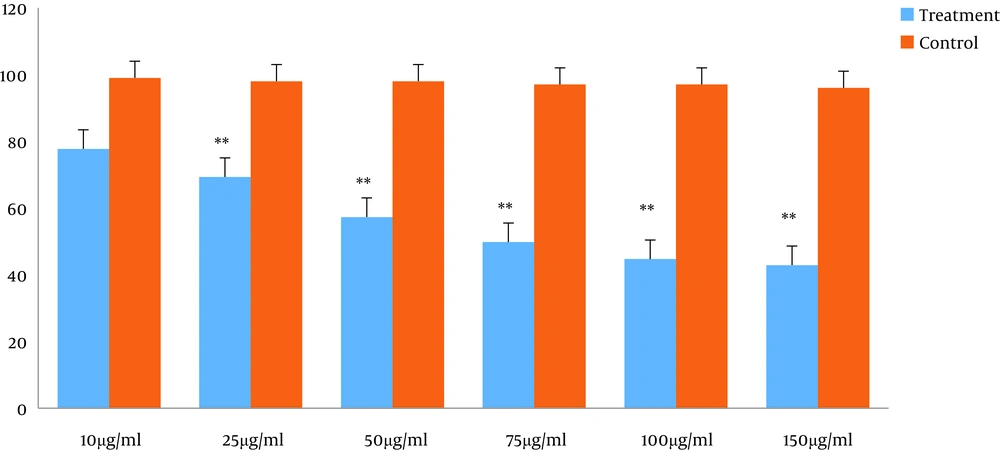
To investigate the potential effect of TpEO, the cells were exposed to the TpEO at concentrations of 0, 10, 25, 50, 75, 100, and 150 µg/mL for 48 h. The calculated IC50 for the HT29 cell line was 66.867 ± 1.37 µg/mL after 48hours (Figure 1).
4.2. Sub-G1 Apoptosis
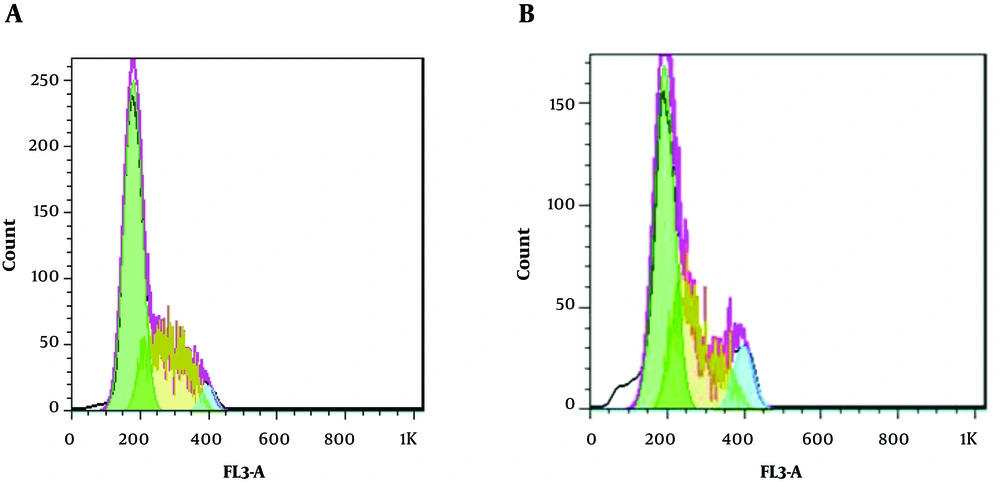
66.867 µg/mL of the TpEO was used in flow cytometry. As shown in Figure 2, when cells were treated with TpEO, sub-G1 apoptotic content was significantly increased.
Sub-G1 was formed in 0.64% of cells (Figure 2A), whereas in the absence of the TpEO, its value was equal to 59.89% (Figure 2B).
4.3. Annexin V Assay
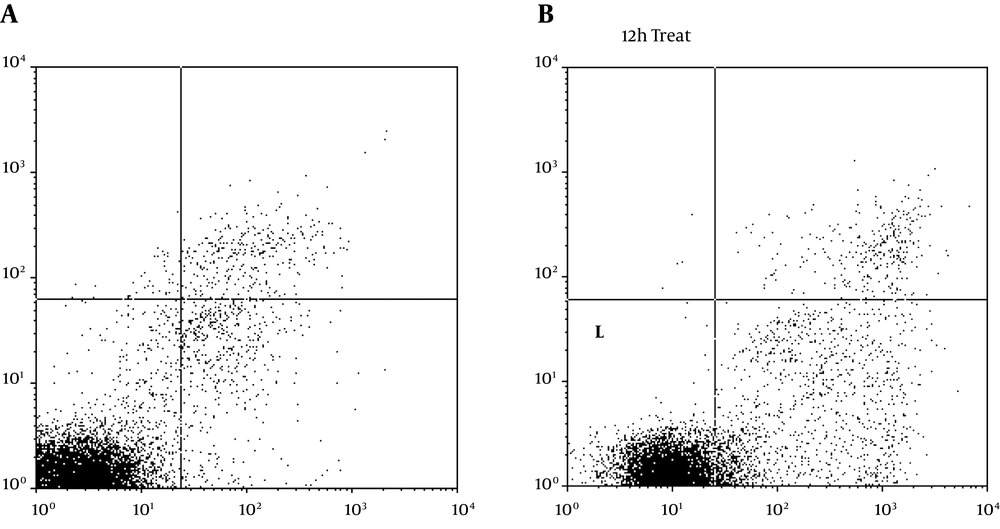
As a result of treating with the TpEO, cancer cells developed many of the hallmark features of apoptosis, including phosphatidylserine exposure on the cell surface (Figure 3).
After 48 h, 48.89% of the cells were shown to expose phosphatidylserine, when the proportion of annexin V bound cells was quantitated.
4.4. Anti-Mutagenic Effects of TpEO
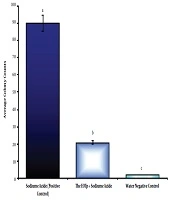
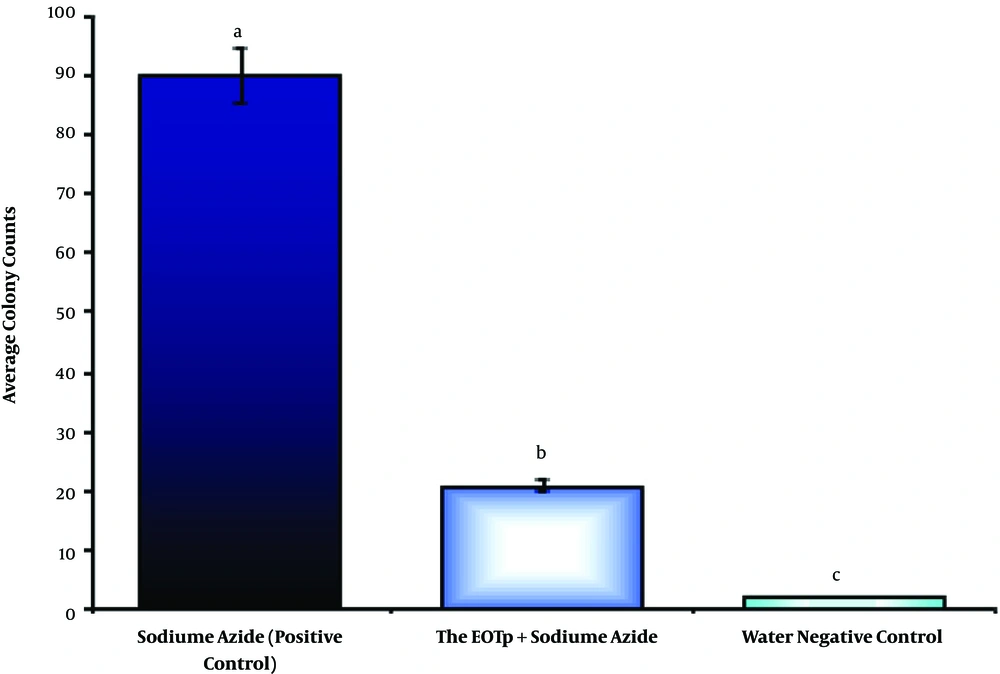
Colony counting in the Amest test under 66.867 µg/mL of TpEO showed a significant difference between the TpEO and control groups in the antimutagenic effect (distilled water and sodium azide) (P < 0.01).
The hindrance percent of TpEO was 78.18% in the anti-mutagenicity, and TpEO prevented the reverted mutations and test (Figure 4), in the Amest test.
5. Discussion
Recently, the tendency to discover novel natural agents, particularly plants, to treat and prevent various types of cancers has increased (21-23).
A review by Ben-Aryea et al. (24) reported that, especially in the Middle East, research activities on CAM (complementary/alternative medicine) in the field of oncology are expanding. Several studies published on the effects of herbs to treat or prevent cancer clearly reveal this trend.
In this study, the main constituents of the essential oil of T. polium were 10-epi-γ-Eudesmol, spathulenol, elemol, α-pinene, and β-caryophyllene. Previous studies on T. polium also reported these compounds, but at various concentrations. In a study by Ashnagar et al. (25), in the Khuzestan province of Iran, the main components of T. polium are reported as γ-muurolene, limonene, α-pinen, β-Eudesmol, β-caryophyllene, and γ-elemene.
Mahmoudi and Nosratpour (26) reported linalool, Germacrene-D, spathulenol, Germacrene-β, β-pinene, and β-myrcene as the main components of TpEO were.
Some components of the essential oil of T. polium have cytotoxic and antiproliferative properties on different cell lines. For example, α-pinene has antiproliferative and cytotoxic effects on N2a neuroblastoma cells (27). The calculated IC50 for the B16-F10 and K562 cell lines were 8.86 ± 1.27 and 15.15 ± 1.06 µg/mL, respectively. All eudesmol isomers (α-eudesmol, β-eudesmol, and γ-eudesmol) showed cytotoxic activities in different cancer cell lines (28).
In a study on the cholangiocarcinoma cell line, Kotawong et al. (29) reported that β-eudesmol affected the cell apoptosis, growth inhibition, and cell cycle arrest.
β-caryophyllene and β-caryophyllene-oxide showed a cytotoxic effect against different cancer cells. The findings of Dahham et al. (30) showed that strong growth inhibition in HCT‐116, HT‐29, and two colon cancer cell lines will be led by treatment with b-caryophyllene (30, 31).
Because of its constituents and cytotoxic effects on some types of tumor cell lines, T. polium is considering as a novel anticancer agent (2, 32).
This study showed cytotoxic effects of TpEO on HT29 cells, in a concentration-dependent manner with an IC50 = 66.867 µg/mL. Further analysis demonstrated that HT29 cells treated with T. polium exhibited morphological features of apoptosis.
MTT and sub-G1 apoptosis assay confirmed the cytotoxic effect of T. polium on the HT29 cell line. The percent of prevention for the essential oil of T. polium was 78.18%. In Ames theory, the percent of prevention less than 25%, between 25% - 40% and more than 40% indicate a negative, medium, and strong anti-mutagenic effect of the chemicals, respectively (33, 34).
As shown by Khader et al. (4), ethanolic extract of T. polium has an anti-mutagenicity effect through direct interaction with the mutagen, increased recovery from DNA damage, and induction of detoxifying enzymes, while they reported that the extract did not affect necrosis and apoptosis.
The effect of ethanolic extract of T. polium on cell lines of BT20 (human breast ductal carcinoma), PC12 (mouse pheochromo-cytoma), A549, and MCF-7 is demonstrated by Nematollahi-Mahani et al. (5). Attaining such results is attributed to the presence of some flavonoids, diterpenoids, and selenium in T. polium.
Another study demonstrated that the methanol extract of T. polium in combination with vinblastine, doxorubicin, and vincristine showed an inhibitory effect on the growth of A431 (epidermoid carcinoma), Saos-2 (osteoblastoma), EJ (bladder carcinoma), KB (oral cavity epidermal), Skmel-3 (melanoma), SW480 (colon carcinoma), and MCF-7 (breast carcinoma) cells.
In addition, the apoptotic effects of the combined treatment of vinblastine/Me-TP and vincristine/Me-TP were higher (> 80%) than each drug alone (0% - 3%). Therefore, it’s concluded that the effects of these drugs might be potentiated by combinations of vincristine, doxorubicin, Me-TP, or vinblastine (7).
The antiproliferative effect of essential oils of four Teucrium species was evaluated on amelanotic melanoma (C32), colon adenocarcinoma (CACO-2), and lung carcinoma (COR-L23). The highest antiproliferative effect of oil of T. polium was found on CACO-2 cell lines (IC50 = 52.7 µg/mL). This result was partly attributed to the presence of sesquiterpenes, such as a-humulene, spathulenol, caryophyllene oxide, d-cadinene, caryophyllene, and tor-reyol in T. polium (8).
The aqueous and methanol extracts of T. polium have a cytotoxic effect on REYF-1 cell line (9).
However, there are also studies that rejected the anticancer effects of T. polium. For example, Khader et al. (3) reported no antimutagenic and cytoprotective activity of aqueous extract of T. polium. Also, another study showed that the aqueous extract of T. polium was not cytotoxic in pheochromocytoma and human liver carcinoma cell lines (6).
The observed difference in the results of various studies can be attributed to the administration of various extracts of T. polium. More than 134 constituents, including neoclerodane diterpenoids, monoterpenes, sesquiterpenes, polyphenols, flavonoids, and fatty acids, are characterized from various parts of T. polium subspecies (2). The difference in various compositions can be explained by using different parts of the plant to prepare the extract, geographical variation, and type of extract. To determine the true effects of each extract, it has to be further evaluated by fractionation.
Thirty components were indicated from GC/MS analysis of the essential oil obtained from Teucrium marum, which mainly included α-caryophyllene (7.18%), dolichodial (9.38%), α-santalene (10.97%), α-santalene (10.97%), β-bisabolene (14.73%), and isocaryophyllene (20.24%) (35).
In the study of Menichini et al. (8), 150 fifty components were identified in the essential oils of four Teucrium species. The most abundant components in T. polium ssp. Capitatum were caryophyllene and carvacrol. Also, all oils had 50.1% - 55.8% sesquiterpenes (8).
This study presented the anti-mutagenic effect of TpEO by the Ames test. Although the Ames test is commonly used, it is important to use other statistical tests before reporting the anticancer effects of any compound.
5.1. Conclusions
This study showed that the essential oil of Teucrium polium has antiproliferative and apoptotic effects on the HT-29 cell line. The authors suggest performing further experimental and clinical studies on the effects of TpEO on colon cancer.