1. Background
Idiopathic pulmonary fibrosis (IPF) is an incurable, progressive, fatal lung disease that is associated with alveolar epithelial cell damage, production of pro-inflammatory cytokines, increased fibroblast/myofibroblast proliferation, and excessive deposition of collagen. Pulmonary fibrosis has a poor prognosis that can lead to death within 2 - 5 years after diagnosis (1).
Although the molecular pathogenesis of pulmonary fibrosis is not well-known, it is shown that inflammation and oxidative stress play important roles in the initiation and progression of pulmonary fibrosis (2). The increased oxidative stress markers and inflammatory cell infiltration have been identified in IPF patients (3, 4). Activated inflammatory cells including macrophages, lymphocytes, and neutrophils by the release of a large number of reactive oxygen species (ROS), cytokines, and growth factors can lead to the proliferation of fibroblasts, stimulation of collagen production by myofibroblasts, and subsequently pulmonary fibrosis (5). Therefore, antioxidant and anti-inflammatory agents may have therapeutic potential to treat pulmonary fibrosis.
Currently, FDA-approved drugs for IPF include nintedanib and pirfenidone, which show poor efficacy in most patients (6). Therefore, we need to discover novel drugs with more efficacy and less adverse effects for the treatment of IPF.
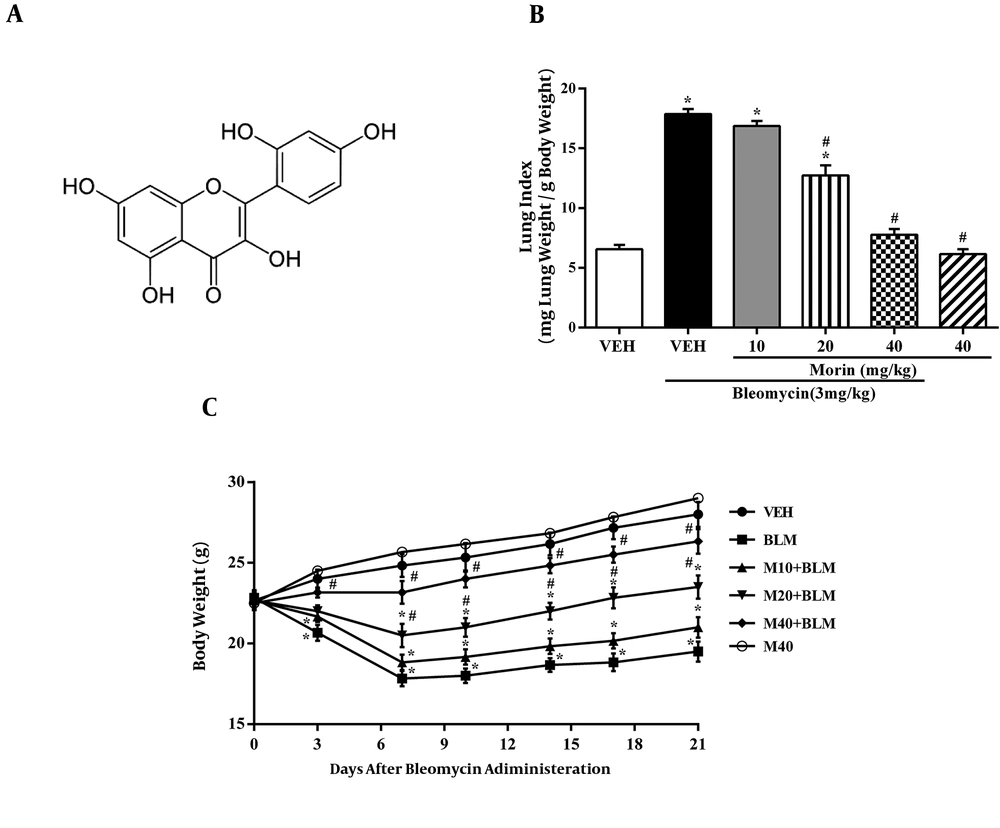
Morin (2’,3,4’,5,7-pentahydroxyflavone; Figure 1C), as a flavonoid, is abundantly found in a number of plants such as Moraceae family members (7). Morin has several pharmacological activities, including anti-allergic, antioxidant, immune regulatory, anti-inflammatory, anti-apoptosis, and anticancer properties (8-11). Recent studies have reported the anti-fibrotic effect of morin in liver fibrosis induced by nitrosamine compounds and CCl4 (12, 13). Moreover, Tianzhu et al. showed that treatment with morin decreased inflammatory responses and oxidative stress markers in LPS-induced lung injury (14).
2. Objectives
Based on the potent anti-inflammatory and antioxidant effects of morin, the aim of this study was to evaluate the protective activity of morin on pulmonary fibrosis induced by bleomycin in mice.
3. Methods
3.1. Animals
Male C57BL/6 J mice (6 - 7 weeks), weighing 20 - 25 g, were obtained from Razi Institute (Karaj, Iran). C57BL/6 J mice were used in this study because they are inbred murine strain sensitive to BLM-induced lung fibrosis. The animals were kept at a temperature controlled room at 23 ± 1°C with access to water and food ad libitum. The study was approved by the Institutional Ethics Committee of the School of Medicine, Ahvaz Jundishapur University of Medical Sciences, and was conducted based on the Guidelines for Animal Care and Use (IR.AJUMS.REC.1395.148).
3.2. Drugs and Chemicals
Bleomycin hydrochloride (BLM) was obtained from Santa Cruz Biotechnology (Santa Cruz, California, USA). Morin, DTNB (5,5’-dithiobis (2-nitrobenzoic acid), TBA (2-thiobarbituric acid), and TCA (trichloroacetic acid) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The activities of superoxide dismutase (SOD) and Glutathione peroxidase (GPx) were assayed with commercial kits (Randox Com, UK).
3.3. Experimental Design
The mouse model of pulmonary fibrosis was induced by a single oropharyngeal administration of bleomycin as previously described (15). We chose the dose of bleomycin at 3 mg/kg dissolved in 50 µL saline according to the results of a preliminary bleomycin dose-response experiment that induced consistent fibrosis but with no fatality. The mice were anesthetized with ketamine hydrochloride (45 mg/kg) and xylazine (9 mg/kg) injections. Then, they received bleomycin on day 0 while mice in the control group received equal amounts of saline (i.t.). The animals were randomly divided into the following groups: Group A (saline + vehicle), Group B (BLM + vehicle), Group C (BLM + morin 10 mg/kg), Group D (BLM + morin 20 mg/kg), Group E (BLM + morin 40 mg/kg), and Group F (saline + morin 40 mg/kg). Morin was dissolved in saline and orally administrated to mice once a day starting from day 0 until day 21. The dose of morin was selected from a previous study (14). Body weights were monitored every three days. On day 21 after bleomycin administration, six mice from each group were euthanized. The left lobe of the lung was placed in formalin for pathological evaluation and the rest was kept at -80°C.
3.4. Bronchoalveolar Lavage Fluid (BALF)
To collect the BALF, the lung was washed five times with 1 mL of phosphate-buffered saline (PBS) using a 1-mL syringe. Then, the BALF was centrifuged at 1500 rpm for 10 minutes and the cell pellet was used for the analysis of inflammatory cells. The cell pellet was suspended in PBS and the total number of cells was counted using a hemocytometer. The percentage of leukocytes were calculated by the counting of about 300 cells stained with Giemsa.
3.5. Lung Index
The lung index was calculated by the lung wet weight (mg) divided by body weight (g).
3.6. Histopathological Examination
Lung tissues were placed in 10% buffered formalin for 48 hours and then embedded in paraffin. Serial paraffin sections (4-µm thickness) were prepared and stained with Masson’s trichrome and hematoxylin-eosin (H&E). The intensity of pulmonary fibrosis was evaluated by the Ashcroft score (16).
3.7. Hydroxyproline Assay
The lung hydroxyproline content was determined according to the colorimetric measurement procedure as reported by Edwards and O’Brien (17). The lung tissue (10 mg) was digested in 1 mL of 6 N hydrochloric acid for 8 hours at 120°C. Then, the buffer solution and chloramine T reagent (1 mL, 0.05 M) were added and the sample was placed at room temperature for 20 minutes. Then, 1.5 mL of aldehyde-perchloric acid was added and the sample was placed at 60°C for 15 min to form a red complex. Absorbance was read at 550 nm by a spectrophotometer. The hydroxyproline concentration was calculated as mg/g of lung tissue by using the equation that resulted from a standard curve of hydroxyproline.
3.8. Measurement of Oxidative Stress Markers
The lung tissue was homogenized in the radioimmune precipitation assay (RIPA) buffer and then centrifuged for 15 min at 3000 rpm. The separated supernatant was used for measuring oxidative stress markers. The GPx and SOD activities were measured by commercial kits (Randox, UK) according to the manufacturer’s instructions. Tissue malondialdehyde (MDA) and glutathione (GSH) levels were measured according to Mihara and Uchiama (18) and Ellman (19), respectively. The protein concentration in the supernatant was measured by the Bradford method.
3.9. Statistical Analysis
The results were presented as means ± SEM. The mean values of different groups were compared with the one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test and the two-way ANOVA followed by Bonferroni’s test. The scores of fibrosis were analyzed using a nonparametric test (Kruskal-Wallis test). A P value of < 0.05 was taken as a significant difference. The GraphPad Prism version 6.01 software was used to analyze the data.
4. Results
4.1. Effect of Morin on BLM-Induced Weight Loss and Lung Index
Mice in the BLM group had significant weight loss compared to the vehicle group during the whole period of the experiment, especially between days 7 and 10 (Figure 1C). The body weight of mice was significantly higher in the morin 20 and 40 mg/kg groups than in the BLM group over the entire experiment period (P < 0.05). There was no significant difference in body weight between the morin-treated (40 mg/kg) group and the vehicle group during the whole experiment (P < 0.05).
As shown in Figure 1B, bleomycin administration resulted in an increased level of lung index compared to the vehicle group (P < 0.05). Lung index levels were significantly lower in the morin 20 and 40 mg/kg groups than in the BLM group (P < 0.05).
4.2. Effect of Morin on BLM-Induced Inflammatory Cells Accumulation in BALF
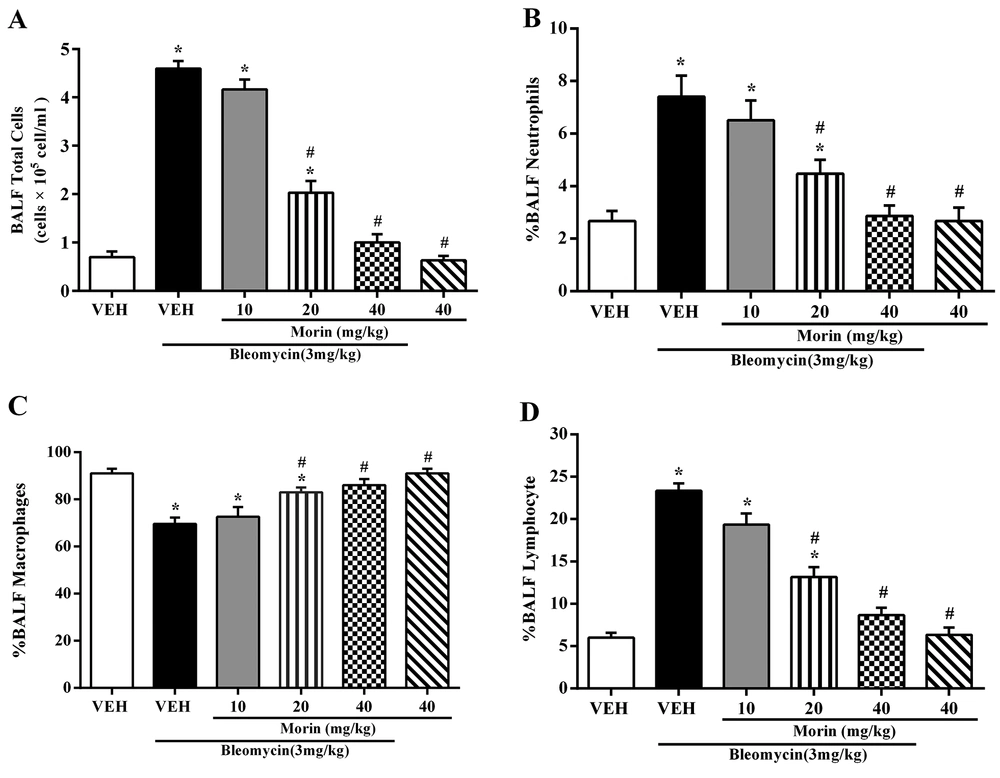
The total number of cells in the BALF markedly increased with bleomycin administration and significantly decreased with morin treatment (20 and 40 mg/kg) (Figure 2A). The percentage of neutrophils and lymphocytes in the BALF significantly increased with bleomycin administration and percentage of macrophages was lower compared to the vehicle group (Figures 2A - 2D; P < 0.05). Treatment with morin (20 and 40 mg/kg) could reverse these effects (Figures 2B - 2D; P < 0.05). There were no significant differences in the total cell number and inflammatory cell percentage between morin-treated (40 mg/kg) and vehicle groups (P < 0.05).
4.3. Effect of Morin on BLM-Induced Lung Histological Changes and Hydroxyproline Content
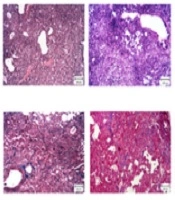
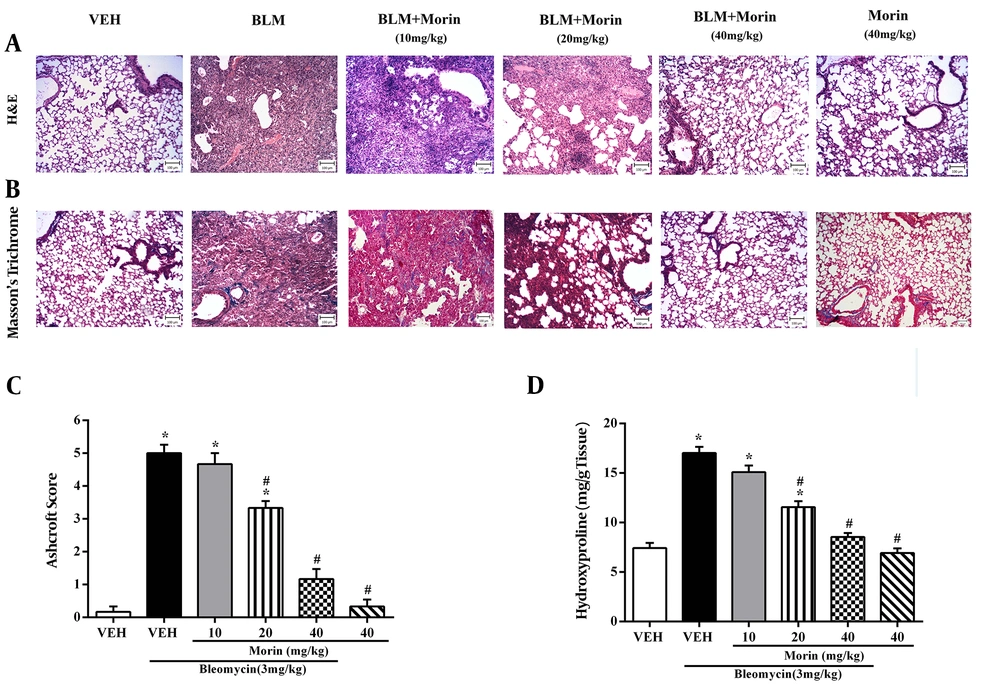
The vehicle group displayed the structure of the normal lung, consisting of a thin alveolar wall and no pathologic changes. The pathological evaluation of lung tissues using H&E and Masson’s trichrome staining showed that bleomycin led to severe lung damage (destruction of alveoli, thickened and edematous alveolar walls, and infiltrations of inflammatory cells with predominant lymphocytes), and collagen deposition in the interstitial space, as indicated by blue staining. However, these effects were mildly ameliorated by morin 20 mg/kg treatment and completely abrogated by morin 40 mg/kg treatment (Figures 3A and 3B). Furthermore, bleomycin administration increased the Ashcroft score and hydroxyproline content compared to the vehicle group (Figures 3A and 3B; P < 0.05). Morin (20 and 40 mg/kg) significantly ameliorated the Ashcroft score and hydroxyproline content compared to the BLM group (P < 0.05). Morin (40 mg/kg)-only administrated animals showed profiles similar to the profile of control animals.
Effect of morin (10 - 40 mg/kg) on BLM-induced pulmonary fibrosis in mice. A, H&E staining of lung tissues (100X); B, Masson’s trichrome staining of lung tissues (100X); C, Ashcroft score; D, content of hydroxyproline in lung tissues. The results are expressed as means ± SEM. *P < 0.05 compared to vehicle (VEH); #P < 0.05 compared to bleomycin (BLM).
4.4. Effect of Morin on Oxidative Stress Markers
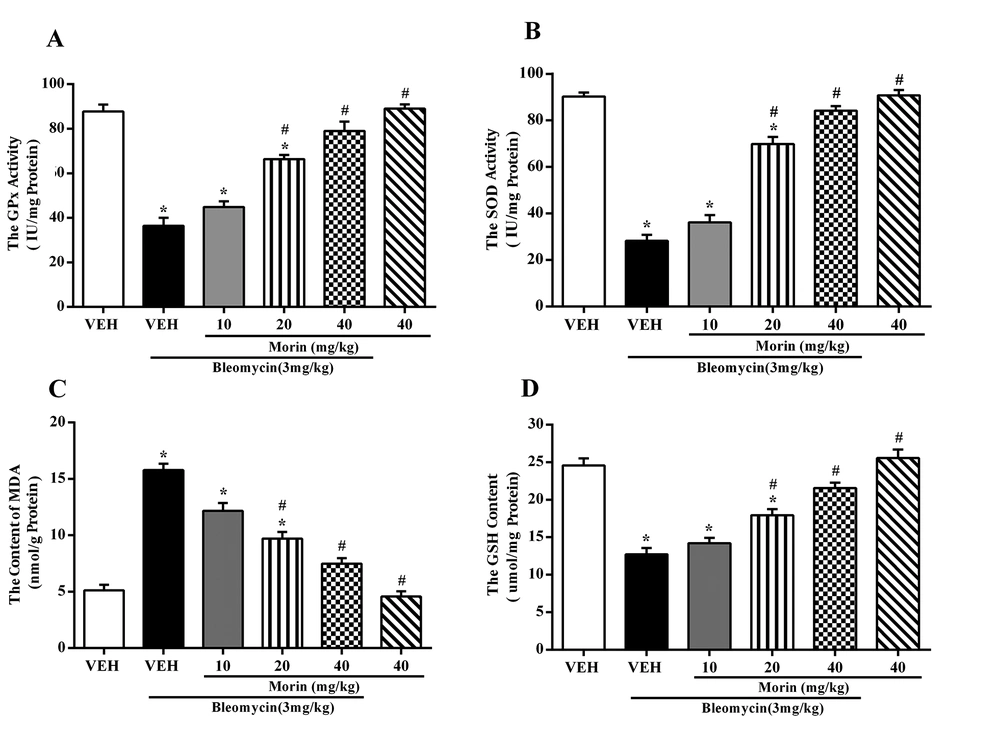
Bleomycin increased the lung MDA content while it decreased the activities of SOD and GPx and GSH level in the BLM group compared to the vehicle group (Figures 4A - 4D; P < 0.05). Treatment with morin (20 and 40 mg/kg) decreased the MDA content and increased SOD and GPX activities compared to the BLM group (P < 0.05). Moreover, morin (20 and 40 mg/kg) treatment prevented GSH depletion induced by bleomycin.
Effect of morin (10 - 40 mg/kg) on the activity of A, GPx; B, SOD; C, the level of MDA; and D, GSH, in lung tissues on day 21 after bleomycin administration (3 mg/kg). The results are expressed as means ± SEM (n = 6 mice). *P < 0.05 compared to vehicle (VEH); #P < 0.05 compared to bleomycin (BLM).
5. Discussion
The results of this study indicated that morin attenuated the development of pulmonary fibrosis in mice in a dose-dependent manner. Treatment with morin significantly decreased collagen production, lung index, inflammatory cells accumulation in the BALF, and lipid peroxidation while they increased in the lungs of bleomycin-instilled mice. The administration of morin also improved the antioxidant enzymes activity in the lung. The protective effects of morin in the bleomycin pulmonary fibrosis model may be due to the inhibition of oxidative stress and inflammation in the lung.
Bleomycin-induced pulmonary fibrosis in mice is a highly reproducible in vivo model to induce consistent pulmonary fibrosis. The bleomycin induces the production of free radicals by binding to iron and DNA and causes DNA, lipid, and protein damage, leading to the activation of inflammatory and fibrotic responses in the lung (20). Thus, we established a bleomycin-induced fibrosis model to evaluate the anti-fibrotic effect of morin. It is reported that BLM-induced weight loss is associated with lung inflammation (21). Our results showed that a single dose of bleomycin significantly reduced body weight in mice while treatment with morin reversed it. The results also demonstrated that morin dose-dependently decreased the lung index, hydroxyproline content, Ashcroft score, and pathological changes induced by bleomycin. The results suggest that morin exerts anti-inflammatory and anti-fibrotic effects in the lung that is consistent with a previous study that showed oral treatment with morin reduced fibrosis score and collagen deposition in diethylnitrosamine-induced liver fibrosis in rats (12).
Oxidative stress is associated with the etiology of pulmonary fibrosis in human and animal models (3). It has been shown that the scavenging of free radicals by antioxidant compounds prevents the progression of lung fibrosis in IPF patients (22). The endogenous antioxidant enzymes (SOD and GPX) are the important members of the antioxidant defense system that is responsible for the detoxification of free radicals in the lung. Glutathione is an intracellular thiol that protects the lung against oxidative stress (23). For the evaluation of oxidative lung injury, the activities of SOD and GPX and the levels of MDA and GSH were measured in lung tissues in this study. In agreement with a previous study, our results showed that bleomycin administration significantly increased the level of MDA and markedly reduced the activity of SOD and GPx and the level of GSH in the lung, which may be due to the production of ROS, increased lipid peroxidation, and excessive utilization of GSH (24). In mice treated with morin (40 mg/kg), the activity of antioxidant enzymes (SOD and GPx) and the level of MDA and GSH returned to normal. These results suggest that morin, via its antioxidant and free radical scavenging activity, may reduce the ROS production and lipid peroxidation and may facilitate the maintenance of thiol status. Our results are consistent with various reports showing that morin is a potent antioxidant agent that can improve antioxidant status in various tissues, including the liver, brain, cardiovascular system, and lung (13, 14, 25, 26). A recent study showed that morin increased protein expression and the activity of antioxidant enzymes and suppressed the ROS generation through the Nrf2/HO 1 signaling pathway in lung fibroblast cells (27). Furthermore, it has been reported that the antioxidant activity of morin is related to hydroxyl groups at positions C-3, C-4, and C-5 in the chemical structure of morin (28). Therefore, it seems that at least part of the beneficial effects of morin against BLM-induced pulmonary fibrosis may be due to its antioxidant activity.
It has been demonstrated that the increased number of inflammatory cells in the BALF is the hallmark of the inflammatory response in the lung and the inhibition of the inflammatory cells influx into the lung may be an important approach for the prevention of pulmonary fibrosis (4). Macrophages and lymphocytes, by the secretion of various cytokines, lead to the proliferation of fibroblasts and ultimately fibrosis. Neutrophils can also cause lung tissue damage and remodeling by releasing proteolytic enzymes (29). Bleomycin-induced pulmonary fibrosis is related to chronic inflammation with the increased number of inflammatory cells in the BALF in the fibrotic phase (23). In agreement with previous studies, the present results showed that bleomycin could induce a significant increase in the number of total cells and the percentage of neutrophils and lymphocytes in the BALF on day 21 after bleomycin administration (30). In addition, the percentage of macrophages, major cell types in the BALF of normal lung, decreased in the BLM group. The co-administration of morin with bleomycin dose-dependently reduced the total number of cells and the percentage of neutrophils and lymphocytes and increased the percentage of macrophages in the BALF. The anti-inflammatory activity of morin has been shown in various animal models including colitis, atherosclerosis, and asthma (24, 31, 32). Previous studies have shown that treatment with morin decreased inflammatory cells infiltration (including macrophages, eosinophils, and lymphocytes) into the BALF in the ovalbumin-induced airway inflammation model and LPS-induced pulmonary inflammation in mice (14, 24). These results confirm the anti-inflammatory effect of morin in the BLM-induced lung fibrosis.
In conclusion, this study indicated that morin exerts the anti-inflammatory and antioxidant effects on bleomycin-induced pulmonary fibrosis in mice. Therefore, morin may be an effective therapeutic treatment for idiopathic pulmonary fibrosis. However, more studies are required to determine the anti-fibrotic mechanism of morin in pulmonary fibrosis.