1. Background
CRC is one of the most commonly diagnosed malignancies that accounts for nearly 9.4% of cancers all around the world. Its incidence is higher in developing countries than in developed countries (1). Evidence suggests an association between the increased risk of cancer and nutrition (2). A number of native plant derivatives, such as flavonoid compounds, could suppress the cancer progression (2). It’s confirmed that the polyphenolic structure of flavonoids that are found abundantly in fruits and vegetables can impede cancer induction in human tissues (3). Flavonoids, such as quercetin, pinocembrin, pinostrobin, apigenin, and galangin have antioxidant, anti-proliferative, and pro-apoptotic properties (4). Genus Euphorbia (Euphorbiacea) comprising only roughly 2000 species and is widespread in Pakistan, India, and Iran. More than 82 species of the Euphorbia are found in Iran (5). Active components of Euphorbia species have different biological activities, including cytotoxic, anti-tumor, antioxidant, antibacterial, anti-inflammatory, and anti-nociceptive (6, 7). The antioxidant properties of some Euphorbia species such as E. helioscopia are shown (8). Euphorbia splendida Mobayen is a plant widely distributed in the west of Iran (9). It contains diterpenoid, triterpenoid, and flavonoid components (10). The essential role of E. esula and E. helioscopia L (as Euphorbia species) in impeding tumor cellular proliferation, apoptosis, and metastasis is shown in vitro (11, 12). The anti-cancer properties of E. splendida on CRC cell line has not been investigated so far. Mutations in the APC gene (a tumor suppressor in the Wnt signaling) can induce cancer, and in colorectal cancer, its levels reduce significantly (13). Numerous studies have revealed that dysfunction of apoptosis can cause pathogenesis and resistance to radiotherapy and chemotherapeutic of cancer cells, including CRC (14). Flavonoids might be able to prevent CRC and cause stimulation of apoptosis following DNA damage, which is an essential mechanism for cancer inhibition (4). PARP can modify different nuclear proteins by poly ADP-ribosylation (15). In vitro and in vivo inhibiting effects of various flavones and flavonols as inhibitors of RARP1 are proved (16). Since cancer cells are sensitive to inhibitors of PARP1 (17, 18), PARP inhibitor therapy can be a new therapeutic option for pathological conditions such as cancers (19).
2. Objectives
The current study aimed to compare and evaluate the effects of EEE, EEF, doxorubicin, EEE plus doxorubicin, and EEF plus doxorubicin combination on cell death of human colon cancer HT-29 cell line. APC gene expression and PARP protein level determination were studied to obtain first-hand biological data to assess whether APC gene expression and PARP protein level changes are involved in cell death of the HT-29 cell line after treatment with EEE, EEF, and doxorubicin.
3. Methods
3.1. Extraction of E. splendida Extracts
Fresh aerial parts of E. splendida were collected in May 2015 from Arak, Markazi province, Iran, and then were identified by Noori et al. (9). It was deposited under code number CMK10 in the Herbarium of the biology department at Arak University. The sample of the plant was dried under shadow. The powdered materials (900 g) were extracted three times, with 90% ethanol by maceration at room temperature. Different fractions of extract were obtained by liquid-liquid fractionation, using hexane, chloroform, ethyl acetate, and water. The extract and fractions were concentrated by a rotary evaporator.
3.2. Determination of Total Flavonoid Content of the Extract and Fractions
TFC of E. splendida extract and fractions were estimated using AlCl3 colorimetric assay. 20 mg of each sample (and/or quercetin as the standard), already dissolved in 90% ethanol, was mixed with 2.5 mL of 2% AlCl3 solution in 90% ethanol. After 40 min, the absorbance of the produced yellow solution was measured at 425 nm. TFC (as μg quercetin equivalents/mg of sample) for each sample was computed on the basis of a calibration curve using quercetin: (y = 0.0169 x + 0.3526), r2 = 0.995.
3.3. Cell Culture
The tumor HT-29 cell line was obtained from the Pastor Institute of Iran (Tehran, Iran). The cells were preserved in a humidified atmosphere at 37ºC under 5% CO2 in a DMEM with 10% fetal bovine serum, 1% penicillin, and streptomycin. Cultures at ~80% confluence were routinely split 1:5 in 10 cm culture dishes, as noted in the following. The cells were washed twice in pre-warmed PBS. Trypsin-EDTA 0.25% was added to dishes and incubated at 37ºC for 1 - 5 minutes. The cells were detached from the dishes, 1 mL pre-warmed culture medium was added, then transferred to a falcon tube. Cells were centrifuged at 1500 rpm and plated in new dishes with fresh culture medium.
HT-29 cell line was harvested and seeded into 96-well plates at a density of 10000 cells per well for further experiments. Cells were counted before and after being seeded in the 96-well plates. Cells were stained with trypan blue and counted using neo bar lam.
3.4. Cell Viability
MTT test was performed in triplicate for both controls and treatments. HT-29 cell line was treated with different concentrations of 0.01, 0.1, 1, 10, 50, 100, 200, 500, and 1000 μg/mL of EEE, EEF, doxorubicin, and combined of EEE + doxorubicin and combined of EEF + doxorubicin (20). To dissolve and prepare different concentrations of the extract, DMEM was used. The control group contained six wells for each dilution without extracts, and the wells were kept for 24, 47, and 72 hours at 37ºC and 5% CO2 separately. The culture medium was exchanged with an equal volume of fresh 100 mL/L medium. In vitro, morphological changes of cells were observed by an inverted microscope after treatment. After overnight incubation, 10 μL MTT solution was added to each well and incubated at 37ºC and 5% CO2 for 3 hours, then 100 μL DMSO solutions was added. The plates were placed in a shaker incubator and shacked at 37ºC for 10 minutes. Subsequently, the optical density (OD) of the wells was obtained at 570 nm wavelength with a microplate reader. The mathematical methods were used to draw a dose-response relationship polygon curve. Rates of cell viability were computed using the following formula:
Cell viability inhibition rate = (average OD value of control wells - average OD value of testing wells)/average OD value of control wells.
3.5. PARP Assay
PARP concentration of the control and treated HT-29 cells 100 µg/mL of EEE, 1000 µg/mL of EEF, 500 µM of doxorubicin, (200 µg/mL EEE + 200 µM doxorubicin), and (500 µg/mL EEF + 500 µM doxorubicin) concentrations were recognized using a PARP ELISA kit (Biocompare, USA) according to the manufacturer’s instruction with an intra-assay coefficient of variation of < 10% and an inter-assay coefficient of variation of < 10%. HT-29 cells were seeded overnight, treated for 24 hours, and then fixed for detaching adherent cells and were analyzed. The positive and negative controls were cell culture medium and blank, respectively.
3.6. Real-Time PCR Assay of APC Expression
Total RNA of the control and HT-29 cells treated with 100 µg/mL of EEE, 1000 µg/mL of EEF, 500 µM of doxorubicin, (200 µg/mL EEE + 200 µM doxorubicin), and (500 µg/mL EEF + 500 µM doxorubicin) were extracted according to manufacturer’s protocol of TRIZOL reagent (Invitrogen, USA). RNA was stored at -80ºC for the following quantitative real-time PCR. Nanodrop (ND1000) spectrophotometer was used to determine RNA concentrations. Total RNA was converted to cDNA using the Quanti TectRev Transcriptase kit (Qiagen, Germany).
Total RNA was reverse transcribed into cDNA according to manufacturers’ instructions. The reaction was performed in 12 μL volume containing: 4 μL of dNTP mix; 1 μL of RT Random Primers; 1 μL of MultiScribe™ Reverse Transcriptase; 1 μL of RNase inhibitor; to 12 μL of nuclease-free H2O and 1 μg of previously isolated RNA. The reaction was performed in Mastercycler personal (Eppendorf, Germany) with the following thermal profile:
70ºC for 5 min, 42ºC for 5 min, 70ºC for 60 min, 85ºC for 5 min.
Finally, concerning the Real-time PCR, 1 μL cDNA, 10 μL SYBR Green PCR Master Mix, and 1 μM forward and reverse primers were used. Specific primers used for analyzing APC and β-actin RNA (as a housekeeping gene) (20) are shown in Table 1. Reactions were performed on a Rotor-Gene 6000 System (Corbett, Australia) for 40 cycles (95ºC for 20 seconds, 62ºC for 20 seconds, and 72ºC for 20 seconds) after an initial incubation for 5 minutes at 95ºC. Reactions were carried out according to the SYBR Premix Dimer Eraser kit (TaKaRa, Shiga, Japan) protocol.
| Primers | Sequences | Primer Length | Product Size |
|---|---|---|---|
| APC forward | 5’-GGA AGC AGA GAA AGT ACT GGA-3’ | 21 | 114 |
| APC reverse | 5’-CTG AAG TTG AGC GTA ATA CCAG-3’ | 22 | |
| β-actin forward | 5’-AGT CTT CCT TCC TGG GCAT-3’ | 19 | 213 |
| β-actin reverse | 5’-CAG GAG GAG CAA TGA TCT-3’ | 18 |
Measurements were performed in duplicate. The single peak of the melting curve of the target gene showed a strong specificity for the amplification. Differences in the threshold cycle (CT) values of the target gene with corresponding internal control β-actin gene were calculated (ΔCT = CT gene - CT β-actin). The relative expression level of the target gene to β-actin was described using the equation 2-ΔCT.
3.7. Statistical Analysis
The graph pad prism was used to perform statistical calculations. Data were described as mean ± SD and were analyzed using the one-way analysis of variance (ANOVA) and Tukey HSD post hoc test. Each experiment was tested in triplicate. P < 0.05 was considered statistically significant.
4. Results
4.1. Total Flavonoid Content
TFC values of E. splendid extract and fractions were presented as mean ± SD. The results are shown in Table 2.
| Extraction | Ethanolic Extract | Ethyl Acetate | Hexanic | Chloroformic | Aqououes |
|---|---|---|---|---|---|
| Total flavonoid concentration | 28.1 ± 0.7 mg/g | 102.36 ± 0.67 mg/g | 27 ± 1.7 mg/g | 22.4 ± 0.6 mg/g | 14.11 ± 0.5 mg/g |
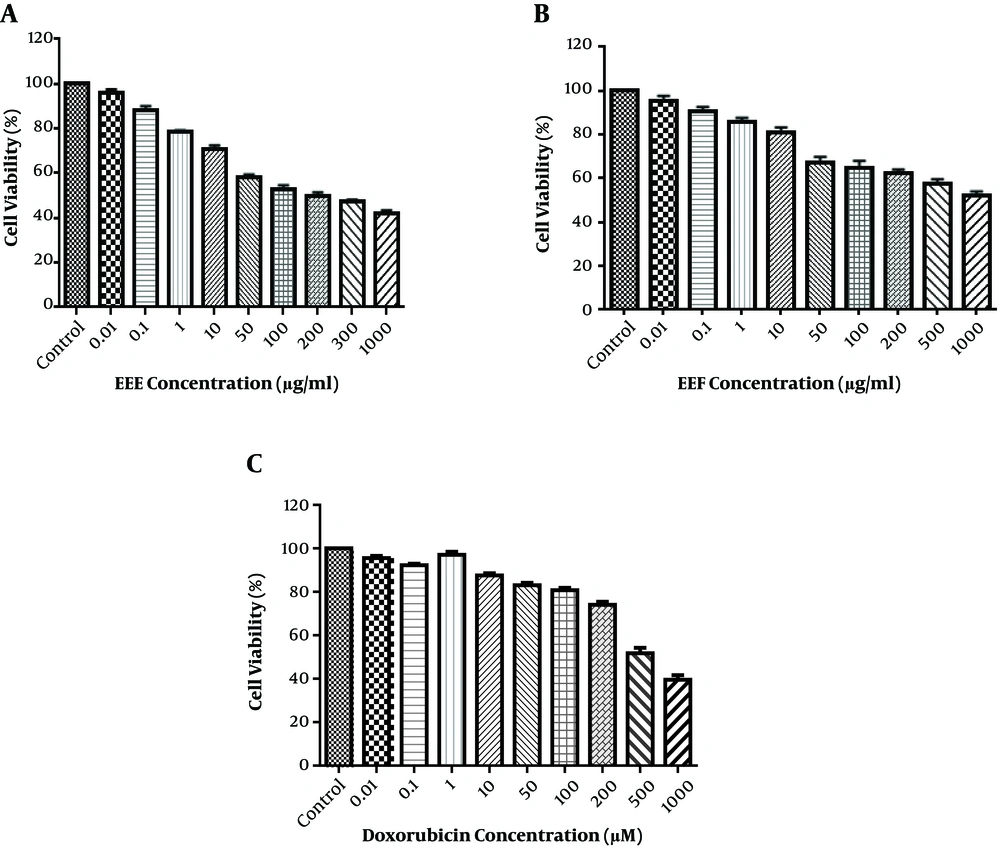
Since EEE and EEF had the highest flavonoid content, these were used for further investigation. Cell viability was identified in the HT-29 cell line, which exposed the control wells to 0.01 - 1000 µg/mL of EEE and Euphorbia ethyl acetate fraction (EEF) for 24 hours of MTT test. As shown in Figure 1A and B, EEE and EEF could cause a dose-dependent decrease in the cell viability. The HT-29 cell viability was reduced to 50% after treatment with 100 µg/mL of EEE and 1000 µg/mL of EEF for 24 hours. The time course was 24, 48, and 72 hours and the ideal treatment time was 24 hours. HT-29 cell treatment with different concentrations of doxorubicin was also investigated to evaluate the cytotoxic effect on HT-29 cell viability. As shown in Figure 1C, doxorubicin-induced a remarkable cell death at a concentration of 500 µM. Therefore, the 500 µM, 100, and 1000 µg/mL concentrations were identified for IC50 of doxorubicin, EEE, and EEF, respectively.
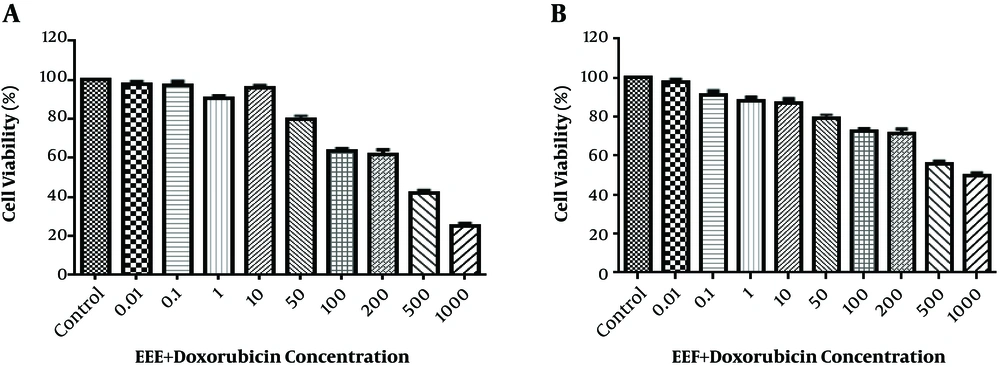
According to Figure 2A and B, the HT-29 cell viability reduced to 50% after treatment with 200 µg/mL EEE + 200 µM doxorubicin combination and 500 µg/mL EEF + 500 µM doxorubicin combination within 24 hours.
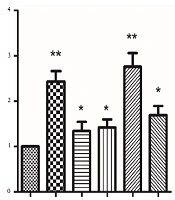
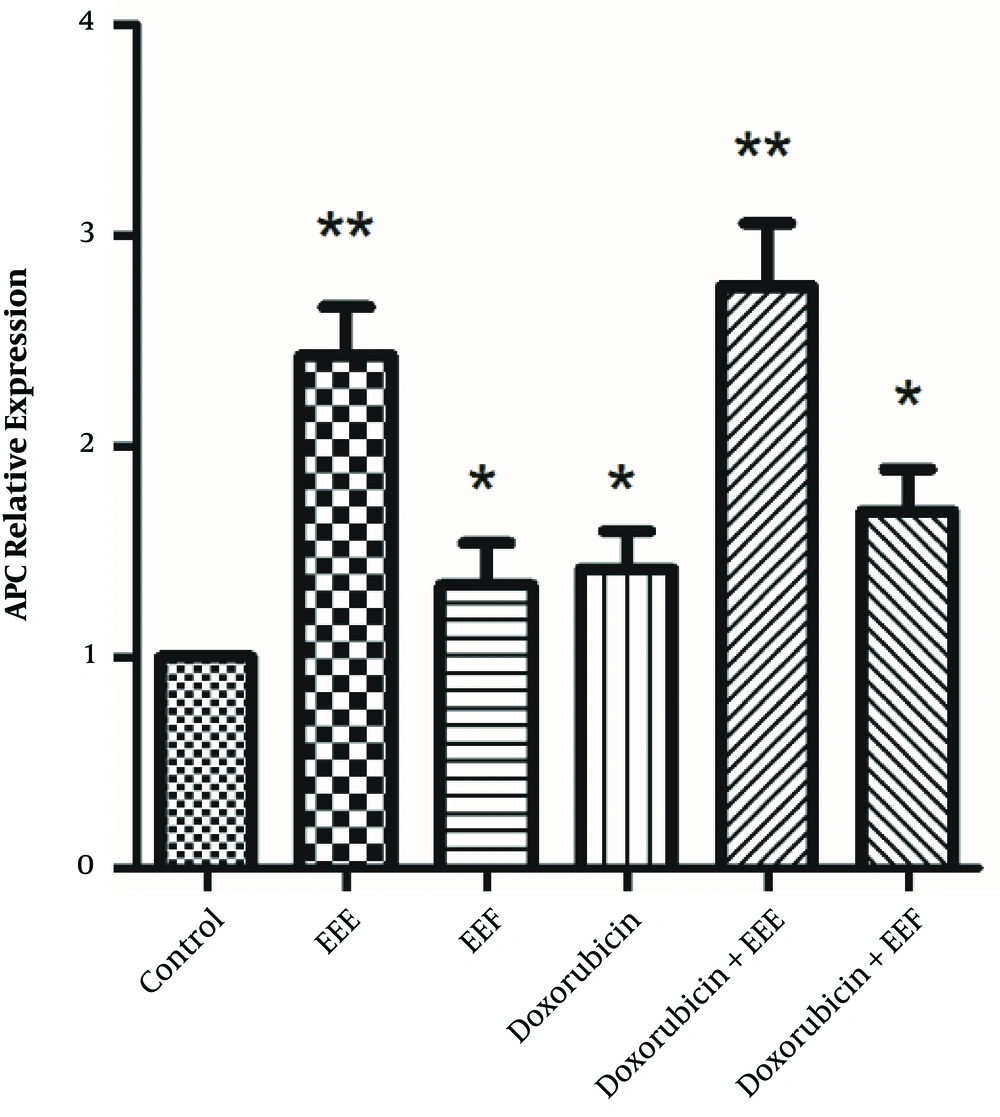
To ensure whether EEE and EEF-induced cell death was caused by enhanced expression of the APC gene, both groups were exposed to 100 µg/mL EEE, 1000 µg/mL EEF, 500 µM doxorubicin, 200 µg/mL EEE + 200 µM doxorubicin combination, and 500 µg/mL EEF + 500 µM doxorubicin combination within 24 hours. The expression levels of internal control and APC genes were determined by the quantitative real-time PCR. As illustrated in Figure 3, APC mRNA level of the treated HT-29 cells within 24 hours was significantly increased compared to controls, for EEE (fold = 2.43, P < 0.001), EEF (fold = 1.34, P < 0.041), doxorubicin (fold = 1.42, P = 0.019), EEE-doxorubicin combination (fold = 2.76, P < 0.001), and EEF-doxorubicin combination (fold = 1.69, P < 0.001).
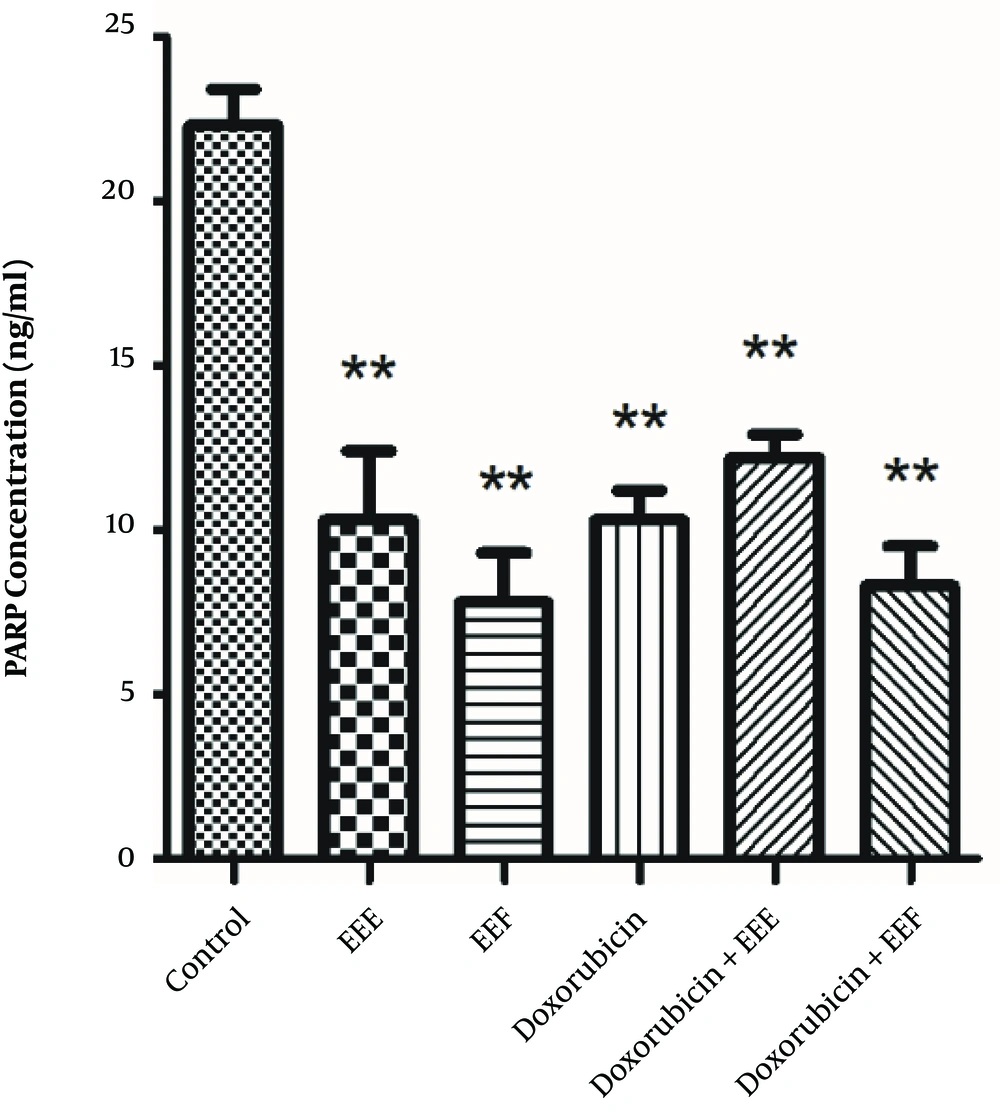
To establish whether changes in the PARP level affect the EEE-induced loss of cell viability, the PARP level of exposed HT-29 cells to EEE, EEF, doxorubicin, EEE-doxorubicin combination, and EEF- doxorubicin combination was examined by the ELISA assay. The PARP level of HT-29 cells was significantly decreased in the presence of 100 µg/mL EEE (P = 0.000), 1000 µg/mL EEF (P < 0.001), 500 µM doxorubicin (P < 0.001), (200 µg/mL EEE + 200 µM doxorubicin) combination (P < 0.001), and (500 µg/mL EEF + 500 µM doxorubicin) combination (P < 0.001) compared to the control cells (Figure 4). The PARP level of exposed HT-29 cells to EEF was lower than other treated groups.
5. Discussion
The findings of the present study indicated decreased cell viability after treating cells with EEE, EEF, and doxorubicin. Accordingly, an inverse linear association was found between flavonoids and the risk of developing cancer (21). Kern et al. suggested that polyphenolrich apple juice extract has strong EGFRinhibitory properties and an anti-proliferative effect on HT-29 (22). Another study demonstrated that flavonoids of red wine have positive effects on individual human organs and methylate the biological compounds to yield products with potentially protective effects against cancer (23). Also, the anti-cancer effects of isoquercetin on colon cancer cells have been proved, whereas it has no significant influence on nontumor colon cells (24). Woo et al. reported that flavonoids can decrease the risk of developing gastric cancer. They also reported greater protecting effects of flavonoids in women (25). Moreover, evidence indicates that Morus alba flavonoid extract induces dose-dependent cell death in the HT-29 cell line (20).
The findings also showed that the PARP concentration of cancer HT-29 cell line decreased after treatment with EEE, EEF, and doxorubicin. EEF induced the highest decrease in the PARP concentration of exposed HT-29 cells. It appears that some exposed efficient groups of EEF could significantly induce PARP level reduction and the observed decrease was higher compared to cells exposed to doxorubicin (26).
A study showed that the PARP activity was inhibited by natural and glucosyl flavonoids that could induce synthetic lethality in BRCA mutant cells (16), which is consistent with the results of the current study.
Recently, PARP1has been shown to influence the repair of DNA and therefore, is considered as targeted therapy (19). PARP1 expression is correlated with a cancer diagnosis (27). PARP1 and its product, PAR, correspond to a variety of endogenous and exogenous stresses that are produced by oxidative, metabolic, and inflammatory stresses (19). These responses can stimulate pathological conditions such as cancer and autoimmune diseases. Therefore, PARP inhibitors can be followed upon as a new therapeutic option for such pathological conditions (19). ABT-888 (Veliparib), an oral PARP-1/2, is a RARP inhibitor being investigated to treat different cancers (28, 29).
Ionizing radiation affects DNA structure by inducing breaks and therefore causes severe DNA damage that leads to cell death. To avoid such side effects, an effective dose of radiation should be administered (30). The combined use of radiation therapy and PARP inhibitors have a greater impact on the tumor cells since the inhibitors stimulate a mechanism that generates double-strand breaks in DNA from the single-strand breaks produced by the radiotherapy in tumor tissue with BRCA mutant. This combination can cause a more potent therapy to target tissues while using a similar radiation dose (31). PARP inhibitors have various functions, and their application is not limited to their efficiency in treating malignancies. During the past decade, a series of functions are identified for PARP inhibitors that integrate the treating of other health problems such as cardiovascular diseases, stroke, metabolic disorders, diabetes, and auto-immunity (32). We supposed that reducing PARP concentration and increasing the APC expression cause inhibitory effects on HT-29 cancer cell growth. Therefore we intended to investigate the effect of these compounds on APC expression and PARP level of HT-29 cancer cells. As shown in Figure 3, all treated HT-29 cells induced the APC level expression compared to the controls. Increased APC expression in treated HT-29 cells can be attributed to the possible anti-proliferative activity of EEE, doxorubicin, and their combination. Accordingly, the elevated APC expression level of exposed HT-29 cell to EEE was more than those exposed to EEF, which might be related to effects caused by other compounds of EEE such as terpens. In 80% of CRC cases, the APC tumor suppressor gene is altered (33). The effect of combined treatment with EEE and doxorubicin on the APC expression level is greater than the sole administration of each extract (Figure 3). It is suggested that EEE can enhance the sensitivity of HT-29 cells to doxorubicin by increasing the APC expression as a possible mechanism of inducing apoptosis and can be used as a co-chemotherapeutic agent with doxorubicin in cancer cell therapy.
5.1. Conclusions
Based on the findings of the current study, it can be recommended that total extract, flavonoid-rich fraction (ethyl acetate fraction), and doxorubicin may have cytotoxic effects probably through stimulating apoptosis. This finding is valuable for identifying other chemopreventive agents. However, further in vivo pre-clinical studies with appropriate animal models are needed. Besides, before clinical testing of EEE as a cancer preventive or a therapeutic agent, special caution should be taken when designing pharmacokinetics studies.