1. Background
Lung tissue is one of the most sensitive organs to ionizing radiation. The main side effects of lung exposure to radiation include acute inflammation (pneumonitis) and late fibrosis, which may appear months to years following exposure (1). These side effects may pose a threat to the lives of patients who had undergone radiotherapy for chest cancer as well as for people who have been exposed to an accidental nuclear or radiological event (2, 3). In recent years, several studies have been conducted to develop effective agents for better amelioration of radiation injury (4-6). Amifostine is an FDA-approved radioprotector for the management of xerostomia in patients with head and neck cancer (7). However, its radioprotective effect is limited to some organs (8). In addition, its high toxicity is the main limiting factor for clinical applications. Some studies reported termination of the radiotherapy procedure, resulting from high toxicity of amifostine (9). In this situation, amifostine treatment may lead to the reduction of therapeutic outcome due to the repopulation of tumor cells (10). Hence, for effective alleviation of the complications to normal tissues, it is necessary to develop low toxic agents with suitable radioprotective effects (11). It is also important that these agents do not interfere with the eradication of cancer cells by ionizing radiation (12).
Knowledge of the mechanisms involved in radiation-induced lung injury can aid the development of new compounds for better radioprotection of injured organs (13, 14). Studies have proposed that mechanisms of radiation injury can be various in different organs. Emerging evidence from published studies have shown that there is an important interrelationship between inflammatory responses and reduction/oxidation (redox) interactions, which mediate radiation toxicity in several organs (15, 16). However, signaling pathways for these interactions may be different. It has been confirmed that an increased level of both inflammatory and fibrotic cytokines such as IL-1, IL-2, IL-6, IL-8, IL-4, IL-13, IL-33, TNF-α, TGF-β, and IFN-γ are involved in the late effects of lung injury by ionizing radiation (17). On the other hand, it is well-known that these cytokines, through upregulation of genes involved in the redox system such as NADPH oxidase, COX-2, iNOS, lipoxygenases, and mitochondria, stimulate continuous production of free radicals, including both reactive oxygen species (ROS) and reactive nitrogen species (RNS) (18-22). So far, studies have confirmed the role of some of these genes, such as NADPH oxidase 1 (NOX1), NOX4, COX-2, iNOS, and mitochondria, in radiation lung injury (23). However, the roles of some others, such as dual oxidases (Duox1 and Duox2), remain to be elucidated.
With regards to the above-mentioned points, it is important to target both inflammatory and fibrotic processes, as well as oxidative injury, for effective protection of the lung against ionizing radiation. Curcumin is a potent modulator of immune responses that can alleviate both inflammation and fibrosis (24). On the other hand, L-selenomethionine is a potent antioxidant that has been shown to be more effective for the amelioration of radiation-induced DNA damage (25).
2. Objectives
In the present study, we aimed to detect the regulation of IL-4Ra1, Duox2, IL-13Ra2, and Duox1 gene expression following rat’s lung irradiation and treatment with a combination of curcumin and L-selenomethionine.
3. Methods
3.1. Drug Treatment and Irradiation
Both curcumin and L-selenomethionine were purchased from Sigma Aldrich (USA). L-selenomethionine was dissolved in distilled water to form a concentration of 0.16 mg per each milliliter. Curcumin was dissolved in 20% ethanol at a concentration of 30 mg per each milliliter. Treatment began a day before radiation exposure. L-selenomethionine was administered through intraperitoneal injection (IP) with a dose of 0.8 mg/kg (26). Immediately, curcumin was administered orally in a 150 mg/kg body weight (27). The protocol was continued for five consecutive days. Prior to irradiation, the rats were anesthetized using a combination of Ketamine and Xylazine for fixation under the source of gamma rays. Irradiation was done with 15 Gy from a Cobalt-60 gamma source at a dose rate of 109 cGy/min in the supine position (PA) in a field size 6×6 cm. The other field size area was shield using lead block.
3.2. Experimental Design
This study involved 4 groups of 5 rats in each group, including G1: control: this group did not receive any radiation or drug treatment, including curcumin and L-selenomethionine; G2: treatment with curcumin and L-selenomethionine: this group received both curcumin and L-selenomethionine at 150 mg/kg and 0.8 mg/kg for five consecutive days; G3: irradiation: this group only received 15 Gy gamma rays to their chest; and G4: irradiation plus curcumin and L-selenomethionine: this group received curcumin and L-selenomethionine 24 hours before irradiation and five consecutive days afterward. On the day of irradiation, both curcumin and L-selenomethionine were administered 30 minutes before exposure to radiation. Sixty-seven days after irradiation, all rats were sacrificed, and their lung tissues were removed after chest opening. The Lungs were frozen at -70°C for Real-time PCR.
3.3. Real-time PCR
The lung tissues were homogenized in TRIzol solution (Takara, Japan), and then total RNA was obtained. Then, cDNA was synthesized for all samples using a thermocycler device and a cDNA Synthesis Kit (GeneAll, South Korea). The primers used in this study were first designed using the Generunner software, followed by blasting all sequences in NCBI for confirmation. The sequence of primers is shown in Table 1. Real-time PCR was done using Applied Biosystems real-time PCR (USA). Moreover, PGM1 was chosen as the internal control gene or housekeeping.
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| IL-13Ra2 | TCGTGTTAGCGGATGGGGAT | GCCTGGAAGCCTGGATCTCTA |
| Duox1 | AAGAAAGGAAGCATCAACACCC | ACCAGGGCAGTCAGGAAGAT |
| IL-4R1 | GAGTGAGTGGAGTCCCAGCATC | GCTGAAGTAACAGGTCAGGC |
| Duox2 | AGTCTCATTCCTCACCCGGA | GTAACACACACGATGTGGCG |
| PGM1 | CATGATTCTGGGCAAGCACG | GCCAGTTGGGGTCTCATACAAA |
Real-time PCR was performed in duplicate, and the amplifications were done using Master Mix Green (Ampliqon). Real-Time PCR efficiency for all genes, including Duox1, Duox2, IL4Ra1, IL13Ra2, and PGM1, was calculated using a linear regression described by Pfaffl (28).
3.4. Statistical Analysis
In this study, we used SPSS software version 24 for all statistical analyses. A student t-test was performed to evaluate significant differences in gene expression. For all analyses, a P-value < 0.05 was considered statistically significant.
4. Results
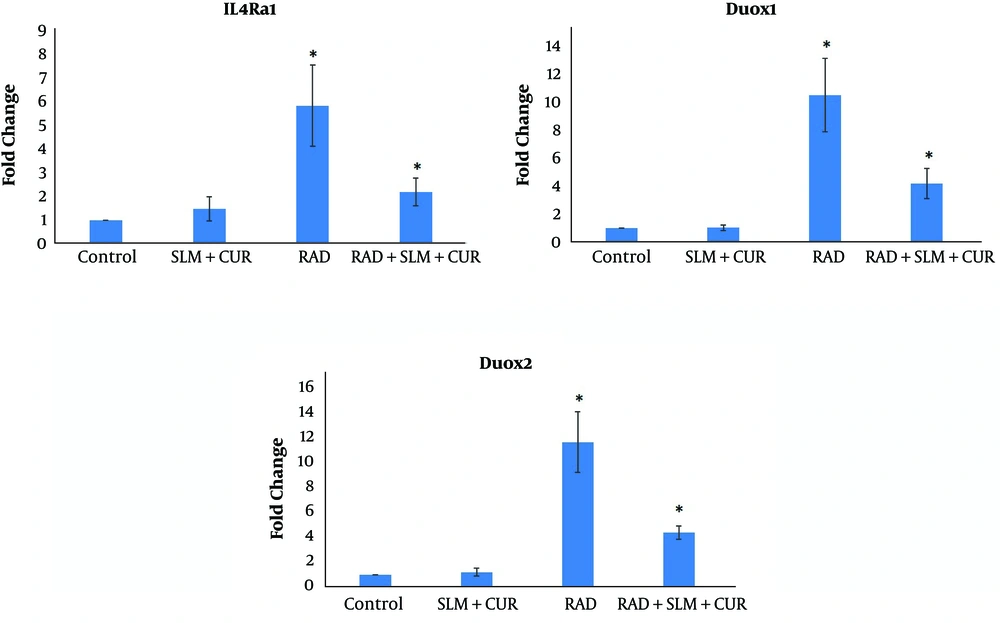
The results of IL4Ra1 gene expression showed that when rats’ lung tissues received irradiation by gamma rays, the expression of this gene increased by 5.94 ± 1.74 fold compared to the control group (P < 0.05). When rats were treated with a combination of curcumin and L-selenomethionine, the expression of IL4Ra1 was reduced by 2.21 ± 0.59 fold compared to the non-treated irradiated rats significantly (P < 0.05). Treatment with a combination of curcumin and L-selenomethionine did not cause any significant change in the expression of IL4Ra1 compared to the control group (1.48 ± 0.52). Real-time PCR results showed no detectable expression for IL13Ra2. The expression of Duox1 when rats received gamma rays to lung tissues was increased by 10.24 ± 2.61 fold compared to the control group (P < 0.05). When rats received a combination of curcumin and L-selenomethionine, the expression of Duox1 was attenuated 4.13 ± 1.07 fold compared to the rats irradiated without curcumin and L-selenomethionine treatment (P < 0.05). Administration of a combination of curcumin and L-selenomethionine alone did not change the expression of Duox1 (1.02 ± 0.19 fold).
The results of Duox2 gene expression showed a significant increase in the expression of this gene following irradiation of rat’s lung tissues (11.70 ± 2.47 fold) (P < 0.05). When rats were treated with a combination of curcumin and L-selenomethionine before and after the irradiation, the expression of Duox2 was attenuated significantly (4.37 ± 0.54 fold) compared to the rats irradiated without treatment (P < 0.05). Similar to other genes, the expression of Duox2 did not change in rats treated with a combination of curcumin and L-selenomethionine alone (1.18 ± 0.32 fold) (Figure 1).
The expression of IL4Ra1, Duox1, and Duox2 following rat’s lung irradiation and administration of a combination of curcumin and L-selenomethionine. A, The radiation group was compared to the control group; and B, the radiation plus treatment group was compared to the radiation group, *, P < 0.05.
5. Discussion
Emerging evidence in recent years has confirmed that the upregulation of some genes, including pro-inflammatory and pro-fibrotic cytokines, plays a central role in the development of radiation-induced lung injury (15). Some studies proposed that modulation of some pathways, including IL-4 signaling, may help mitigate radiation-induced injury in the lung and other tissues such as the heart (29, 30). In addition, supplements with some antioxidants have confirmed that chronic oxidative damage plays a key role in the late effects of radiation on the lung (17). Ameziane-El-Hassani et al. (31) showed that IL-4 and IL-13 could stimulate the upregulation of Duox1 and Duox2, leading to the continuous production of free radicals following exposure of thyroid cells to radiation. In addition, they showed that the upregulation of these genes is associated with genomic instability, which may increase the risk of carcinogenesis. In this study, we hypothesized that irradiation of rat’s lung tissues might lead to the upregulation of Duox1 and Duox2 gene expression. Also, we hypothesized that the expression of these genes in the lung may be dependent on the IL13Ra2 and IL4Ra1 expressions. Results of our study showed that irradiation of lung tissues led to a significant increase in the expression of IL4Ra1, but did not show a detectable expression for IL-13. Also, results showed an increase in the expression of both Duox1 and Duox2.
In the present study, we detected the modulatory effect of curcumin and L-selenomethionine before and after irradiation on the expression of IL4Ra1, Duox1, and Duox2. The results showed that this combination reduces the expression of all three genes. This may indicate that the combination of curcumin and L-selenomethionine may be useful for the mitigation of radiation-induced lung injury through modulation of pro-oxidant enzymes such as Duox1 and Duox2. Previous studies have shown that curcumin can suppress several inflammatory mediators, including inflammatory cytokines, transcription factors such as NF-κB and STATs, and also pro-oxidant enzymes such as iNOS and COX-2 (32-34). On the other hand, L-selenomethionine has been shown to mitigate radiation-induced injury in some organs such as the kidney, bone marrow, and gastrointestinal system (35, 36). The combination of curcumin and L-selenomethionine may be a potent anti-inflammation and antioxidant compound for amelioration of radiation injury.
5.1. Conclusions
This study showed that exposing rat’s lung tissues to a high dose of ionizing radiation leads to upregulation of IL4ra1, Duox1, and Duox2 gene expression. However, we did not detect the regulation of IL13Ra2. Treatment of rats with a combination of curcumin and L-selenomethionine could attenuate the expression of these genes. These results indicate that upregulation of Duox1 and Duox2 may be involved in the late effect of radiation on the lung tissue. Eventually, our results indicated that a combination of curcumin and L-selenomethionine may be useful for the mitigation of lung injury through modulation of these genes.