1. Background
Cancer is a complex disease, which is incurable in some cases (1). Renal Cell Carcinoma (RCC) usually occurs in people over 40 years and accounts for 3% of all cancers. The incidence of this cancer is two times higher in men than in women. Obesity, overweight, and tobacco use are factors that increase the risk of developing kidney tumors. People working in the heavy metal industry, petrochemical industry, and metal mines are at an elevated risk of developing kidney tumors compared to other people in the community. Several studies have been conducted to explore a new diet rich in phenolic compounds to eliminate this malignancy (2).
Chemotherapy is one of the therapeutic techniques for cancer patients (3). Although the main goal of chemotherapy is to remove tumor cells, chemotherapeutic drugs often penetrate healthy cells and destroy them. In addition, many side effects appear after they are taken, including fatigue, nausea, vomiting, diarrhea, mucositis (inflammation of the oral mucosa), pain, rash, infection, and headache (4). Researchers believe that nutritional factors, herbs, and their effective compounds may affect chemotherapy and thus help in treating cancer. Various natural compounds can improve the effectiveness of chemotherapy by decreasing the resistance to chemotherapeutic drugs, reducing or eliminating the side effects of these drugs (5). On the other hand, one of the greatest limitations of anticancer drugs is the resistance of cancer cells to chemotherapeutic drugs that can be caused by the intrinsic resistance of the tumor to the drug or resistance obtained during chemotherapy and may act to select resistant cells from heterozygous cells. As a result, the treatment process becomes more difficult with the growth of resistant cells (6).
Herbal medicines have been considered nowadays because of lacking the side effects of chemical drugs (7). The polyphenolic compounds have been recognized as anticancer agents. The presence of phenolic compounds in various diets reduces the risk of diseases such as cancer and diabetes (8). There is a strong relationship between the type of diet and its impact on cancer. Hence, diet is an important risk factor for the onset of cancer (9). The polyphenolic compounds have anti-proliferative effects and can trigger apoptosis in cancer cells. Based on molecular studies of phenolic compounds in cancer cells, it has been found that these compounds, through increased intracellular calcium, can induce apoptosis in cancer cells, resulting in reduced tumor size and increased chance of recovery in these patients (10).
Ferulic acid is a phenolic compound found in the cell wall of plants such as rice and barley and the seeds of fruits such as apples, oranges, and grapes. Studies have shown that this compound has antioxidant, anti-inflammatory, anti-diabetic, anti-apoptotic, anti-aging, neuroprotective, and anti-tumor effects (11). The induction of apoptosis is one of the functional mechanisms of anticancer drugs that cause the death of cancer cells.
2. Objectives
Given the scope of studies on herbal treatments and the importance of phenolic compounds in the treatment and prevention of cancer, it seems essential to understand the molecular mechanisms of natural compounds in the treatment of RCC. Therefore, the current study aimed to investigate the anticancer and apoptotic effects of ferulic acid on the renal carcinoma cell line of ACHN.
3. Methods
3.1. Materials
The human renal carcinoma cell line (ACHN) was provided by the Pasteur Institute of Iran. Ferulic acid was purchased from Sigma Company (St. Louis, USA). Annexin V-FLUOS Staining Kit and Bax/ Bcl-2 Expression Assay Kit were purchased from QIAGEN Co.
3.2. Cell Culture
The ACHN cells were cultured in RPMI 1640 culture medium enriched with 10% Fetal Bovine Serum (FBS), 100 U/ml of penicillin, and 100 μg/ml of streptomycin under controlled conditions of 37˚C and 5% CO2. The medium was removed three times a week. Trypsin was used to harvest the cells. When at least 80% of the cells reached sufficient growth in the flask, they were separated from the flask bottom by trypsin and centrifuged at 1,500 rpm for 10 min. The cell deposition was suspended in 2 cc of culture medium, and the cell viability percentage was determined by mixing an equal ratio of trypan blue. Cells with viability percentages of higher than 90% were used for the next experiments. The protocols of this study were confirmed by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1396.796).
3.3. Studying Ferulic Acid Cytotoxicity by MTT Assay
The MTT assay was used to evaluate the proliferation of ACHN cells. This test is a rapid and sensitive method for evaluating cytotoxicity in vitro. To this end, 24 hours before ferulic acid treatment, 5,000 cells were seeded in each well of a 96-well plate. Then, various concentrations of ferulic acid (10, 20, 40, 80, and 160 μM) were added to the wells and remained for different periods (24, 48, and 72 hours) in culture conditions. After the given time elapsed, the supernatant was discarded, and 200 μl of the MTT dye was added and incubated for 4 h at 37˚C. Next, 150 μl of dimethyl sulfoxide (DMSO) was added and shaken with a shaker for 10 min at room temperature. The absorbance was read at a wavelength of 570 nm by an ELISA reader. The results were analyzed using the GraphPad Prism version 6.0 software, and the concentration causing 50% removal of viable cells was considered as IC50.
3.4. Analysis of Apoptosis by Flow Cytometry
To determine the percentage of apoptotic cells in treated cells and make a comparison with the control cell group, cell staining was done with the Annexin V, and Propidium Iodide (PI) dyes using the commercial Annexin V-FLUOS Staining Kit. Thus, cells (5×105) were treated with 30 and 60 μM of ferulic acid concentrations for 72 h and then trypsinized and rinsed with sterile Phosphate-buffered Saline (PBS). The sediment resulting from centrifugation of cells was added to 100 μl of the binding buffer in a microtube. Next, 5 μl of PI and 5 μl of Annexin V were added to the contents of the microtube. The contents and the tube were gently mixed. In the next step, the samples were incubated at room temperature (25˚C) for 15 min in the dark. After that, 400 μl of the binding buffer was added to each tube, and cellular analysis was immediately done by FACScan flow cytometry (Becton Dickinson, America).
Data were analyzed using the device software. The positive points in the two-dimensional curve were divided into four regions from Q1 to Q4, including Annexin V-/PI- (left lower quadrant) for viable cells, Annexin V+/PI- (lower right quadrant) for early apoptotic cells, Annexin V+/PI+ (upper right quadrant) for late apoptotic cells, and Annexin V-/PI+ (upper left quadrant) for necrotic cells. The percentage of cells in each area was calculated by FCS Express software and reported as the percentage to determine the effects of ferulic acid on inducing apoptosis or necrosis.
3.5. Evaluation of Bax and Bcl-2 Gene Expression Using Real-Time PCR
Cells (5×105) treated with 30 and 60 μM concentrations of ferulic acid underwent the real-time PCR. The RNA extraction was performed with the RNase-free DNase kit. The next step was the cDNA synthesis using the cDNA Synthesis Kit (Thermo Scientific Co.). The real-time PCR process was performed in a final volume of 10 μl. Primers, enzymatic mixtures, and DEPC-treated water first reached the ambient temperature and were prepared after short centrifugation. Strip tubes (20-μl tubes connected to each other specific for the real-time PCR) were placed on ice under ventilation and added with 5 μl of 2X Master Mix, 3 μl of DEPC-treated water, and 0.5 μl of each primer. The cDNA was then melted onto the ice, shortly centrifuged, and added to each strip tube. Finally, the samples were transferred into the real-time PCR device. The experiment was carried out after temperature and 45-cycle setting. The primers used in this study are presented in Table 1.
| Primer | Sequence | Primer Length |
|---|---|---|
| Bax F | 5'-AGAGGATGATTGCCGCCGT-3' | 19 |
| Bax R | 5'-CAACCACCCTGGTCTTGGATC-3' | 21 |
| Bcl-2 F | 5'-TTGGCCCCCGTTGCTT-3' | 16 |
| Bcl-2 R | 5'-CGGTTATCGTACCCCGTTCTC-3' | 21 |
| GAPDH F | 5'-TTCTATAAATTGAGCCCGCAGCC-3' | 23 |
| GAPDH R | 5'-CCGTTGACTCCGACCTTCAC-3' | 20 |
Primer Sequences and Lengths Used in the Study
3.6. Statistical Analysis
In this study, the one-way ANOVA test with P < 0.05 was used to compare between all groups and the t-test to compare between two groups. The changes in Bcl-2 and Bax gene expression were calculated by comparing with GAPDH gene expression using the Pfaffl method.
4. Results
4.1. MTT Assay
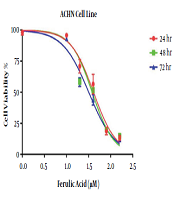
In the MTT assay, the IC50 values of ferulic acid against the ACHN cell line were 39.5, 34.3, and 30 μM at 24, 48, and 72 hours, respectively. Hence, the minimum IC50 for this drug, which was achieved within 72 hours, was used as the IC50 for subsequent experiments. Furthermore, the effects of ferulic acid at the doubled concentration of IC50 (60 μM) were evaluated on the ACHN cell line. The results are shown in Figure 1.
4.2. Flow Cytometry
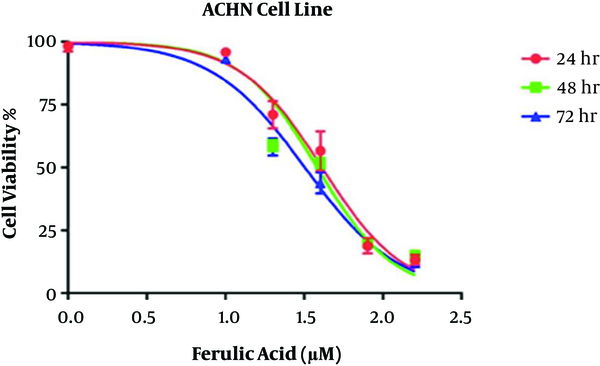
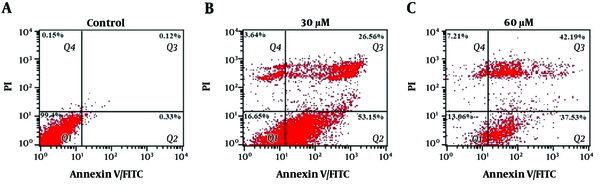
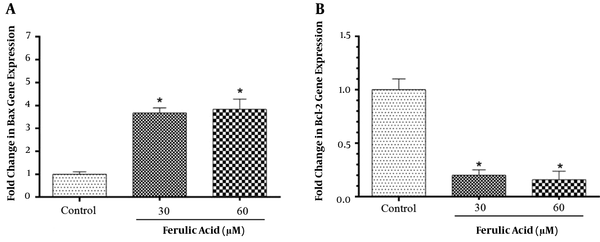
The flow cytometry with Annexin V-FITC/PI staining was used to evaluate the post-treatment apoptosis. The control group, unexposed to ferulic acid, showed 99.4% viable cells, 0.45% total early and late apoptotic cells, and 0.15% necrotic cells. The group treated with 30 μM of ferulic acid showed 16.65% viable cells, 79.71% total early and late apoptotic cells, and 3.64% necrotic cells. The group treated with 60 μM of ferulic acid showed 13.06% viable cells, 79.72% total early and late apoptotic cells, and 7.21% necrotic cells. The results are shown in Figure 2. The Bcl-2 protein family plays a key role in apoptosis, and pro-apoptotic and anti-activity levels are important in controlling apoptosis in cells. The study confirmed the role of Bcl-2 in inducing apoptosis caused by ferulic acid. The effects of ferulic acid on the expression of the Bax and Bcl-2 genes were investigated using real-time PCR in the ACHN cell line. The expression of the Bax and Bcl-2 genes are shown in Figure 3.
Apoptotic cell determination of ACHN cancer cells at different concentrations of ferulic acid by flow cytometry and Annexin V/PI staining, Group A: Control group (without ferulic acid treatment), Group B: Treatment with 30 μM of ferulic acid, Group C: Treatment with 60 μM of ferulic acid, Q1: Viable cells (Annexin V-/PI-), Q2: Early apoptotic cells (Annexin V-/PI+), Q3: Late apoptotic cells and early necrotic cells (Annexin V+/PI+), Q4: Necrotic cells (Annexin V-/ PI+), Analyzing the Expression of apoptosis-associated Genes
5. Discussion
Cancer is one of the main public health problems in developed and developing countries. According to the World Health Organization, 21 million new cases of cancer and 11.5 million deaths from cancer will occur annually around the world by 2030 (12). Renal cell carcinoma is one of the most common cancers, especially in developed countries. They are tumors that originate from epithelial cells of the renal tubules and account for over 90% of kidney tumors. Renal cell carcinoma is the eighth malignancy among the most common cancers in America (13). The induction of apoptosis or programmed cell death is characterized by certain features such as chromatin condensation, DNA fragmentation, membrane buckling, caspase activity, and phosphatidylserine transfer from the inner to outer layers of the plasma membrane, which is one of the useful tools for cancer treatment (14).
Chemotherapeutic drugs can induce apoptosis, often due to DNA damage and oxidative stress. Oxidative stress is a condition in which the imbalance comes from the production of free radicals and antioxidant defense systems (15). Among chemotherapeutic drugs, it will be beneficial to use compounds that act with the oxidative stress system inside the cancerous cell, such as flavonoid family derivatives. In addition, many studies have indicated that some of these flavonoids have specific applications, especially for the treatment of certain cancers. Many of these compounds can also be effective in overall cancer treatment processes through the mechanisms of inhibiting DNA synthesis, modifying ROS production, regulating the cell cycle, and repairing apoptotic pathways (16). Flavonoids are known as potent antioxidants that protect cells from oxygen-derived free radicals (16). They prevent mutations and initiate carcinogenesis. Recently, it has been observed that they can induce apoptosis in cancerous cells (17). Therefore, further studies are needed to identify the molecular mechanism of naturally occurring anticancer compounds, especially plant-derived compounds.
Evidence suggests that ferulic acid can act as an antitumor agent in various human cancers. It has been shown that ferulic acid inhibits 7,12-Dimethylbenz [a] anthracene-induced (DMBA) in breast and skin cancers through anti-genotoxic and antioxidant features, regulating the effects in phase II detoxification cascades and reducing buccal cancer through decreasing the expression of PCNA and Cyclin D1 (18). In addition, it has been reported that ferulic acid, curcumin, chlorogenic acid, and caffeic acid can inhibit skin cancer induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in rats (19). Ferulic acid is expected to reduce ROS (11); however, since the cancerous cell has an acidic region (20), ferulic acid is unable to play a role as an antioxidant but acts mostly as a pro-oxidant (21). Studies show that ferulic acid, along with radiotherapy, can reduce the antioxidant status and increase ROS, lipid peroxidation, and DNA damage (21). One of the reasons for increasing ROS by ferulic acid may be attributed to its effect on P53 activity. Ferulic acid has been reported to inhibit the change in the function of P53 in buccal carcinoma and has a beneficial effect in treating this type of cancer (22). As known, P53 is a tumor suppressor (23), which also plays a role in regulating the expression of many pro-oxidant and antioxidant genes, including catalase, superoxide dismutase, and glutathione peroxidase. Increasing its proper functioning can lead to a loss of redox hemostasis (24) and induce oxidative stress (25).
The results of this study indicated that ferulic acid can prevent renal carcinoma cell line proliferation in a concentration-dependent manner. Ferulic acid has been reported to induce apoptosis in non-small cell lung cancer (NSCLC) by increasing P53, Bax, caspase 3, and GADD45 (26), as well as in the Caco-2 colon cancer cell line, causing a delay in the synthesis phase of the cell cycle and an increase in the expression of CEP2, CETN3, and RABGAP1 genes, which control DNA damage in the cell cycle of the S phase (27). In the present study, it was found that the anticancer effect and apoptotic induction property of ferulic acid can be due to an increase in the expression of the Bax gene and a decrease in the expression of the Bcl-2 gene, which encodes the apoptotic proteins. It has been proven that ferulic acid induces apoptosis through increasing the expression of the Bax and caspase 3 genes and decreasing the expression of the Bcl-2 gene (28).
The safety of ferulic acid should be considered because ferulic acid has a nephrodamaging effect in chronic use (29). It has also been demonstrated that ferulic acid can reduce multi-drug resistance (MDR) to paclitaxel in cancer cell line KB. This effect is induced by decreasing the expression of the ABCB1 and p-glycoprotein genes, inducing apoptosis through increasing the P53 expression, increasing the Bax/Bcl-2 expression ratio, and increasing CDKN1A, ultimately leading to the enhancement of cell cycle arrest in the G2/M phase along with paclitaxel (30). Besides, Bcl-2 is the first known gene associated with apoptosis, which plays a role in tumor formation (31). The members of the Bcl-2 family in mammals can control the internal pathway of apoptosis by inhibiting the release of cytochrome C and other mitochondrial membrane proteins into cytosol. Some members of the Bcl-2 family, including Bax, act as pro-apoptotic agents and proceed to apoptosis by increasing the release of mitochondrial proteins. However, some other members, such as Bcl-2, act as anti-apoptotic factors and cause the inhibitory release of mitochondrial proteins. Indeed, these two groups restrain each other's actions. The balance between their activities usually determines the presence or absence of cell apoptotic induction (31).
In the current study, the treatment of cancer cells with different concentrations of ferulic acid resulted in cell apoptosis as confirmed by flow cytometry. The flow cytometry studies with Annexin V staining in ferulic acid-treated cells indicated the apoptosis and introduction into the apoptotic phase compared to the control group (without ferulic acid treatment). It was also shown that apoptosis is a major cause of cell death and growth inhibition in predicted concentrations. However, a very small percentage of cells had necrosis, but this rate was not significant compared to the percentage of apoptotic cells. The findings of this study revealed that ferulic acid can precede renal carcinoma cells towards apoptosis and prevent their proliferation.
5.1. Conclusion
According to the results obtained in the present study, ferulic acid has apoptotic effects against ACHN cancer cells. These findings can be helpful in the better understanding of ferulic acid anticancer mechanisms, suggesting its use as an alternative or complementary drug for cancer therapy.