1. Background
Vincristine (VCR) is a vinca alkaloid obtained from the plant Catharanthus roseus (1). It is a potent chemotherapeutic agent currently used in the treatment of acute lymphocytic leukemia, Hodkin’s and non-Hodgkin’s lymphoma, as well as solid tumors such as neuroblastoma, sarcomas, and breast cancer. However, its severe side effects, such as neurotoxicity, have limited its clinical applications (2). Common symptoms of vincristine-induced neurotoxicity include pain, tingling, numbness, and decreased sensation in the hands and feet (3). VCR binds to the tubulin protein and induces apoptosis (4). In recent years, many attempts have been made to prepare a new formulation of VCR with low side effects and high therapeutic efficacy. For this purpose, several types of drug delivery systems such as liposomes, microspheres, nanoparticles, and micelles have been exploited, which in some cases, results have been promising (5, 6). Ying Liu et al. prepared a gold nanoparticle of VCR incorporated in liposomes (7). They studied the drug release, apoptosis, and intracellular delivery of the formulation in HeLa cells. Antitumor efficacy and biodistribution of the new formulation were evaluated in the tumor-bearing nude mice. Their results showed that this new formulation produced greater antitumor effects in nude mice and decreased the side effects, as compared with free vincristine sulfate. In another study, a complex of VCR-dextran loaded solid lipid nanoparticles was prepared by Aboutaleb and Dinarvand for drug delivery to the brain. Their results revealed a significant increase in the plasma level of the solid lipid nanoparticles injected to animals as well as a sharply increased concentration in the brains (8). Niosomes are suitable carriers for controlled drug delivery. They can entrap both hydrophobic and hydrophilic drugs in the outer lipid bilayers and interior aqueous phase, respectively. Niosomes constitute of a non-ionic surfactant such as span 60 to give lamellar structures (9). They are biodegradable, biocompatible, bioavailable, non-toxic, and low-cost carriers and, hence, are good candidates for delivery of therapeutic agents to the site of action with decreased systemic toxicity (10).
2. Objectives
In this study, attempts were made to synthesize and characterize a new PEGylated niosomal VCR. This niosomal formulation was characterized physicochemically, and the releasing rate of VCR from the carrier, in vivo neurotoxicity, and therapeutic efficacy were evaluated in a murine model of lymphoma induced by BCL1 clone 5B1b cell line in BALB/c mice.
3. Methods
3.1. Materials
Polyethylene glycol (PEG-3000), cholesterol, span 60, vincristine sulfate, and MTT solution were purchased from Sigma (USA). Ethanol and isopropanol were bought from Merck company (Germany). RPMI-1640 with glutamax, minimum essential medium (MEM) containing glutamine, trypsin/ethylenediaminacetic acid (EDTA), and penicillin/ streptomycin solution were obtained from Gibco BRL. A red blood cell lysis solution was supplied by Biolegend Inc. Anti-mouse CD5 FITC, anti-mouse IgM PE, and rat IgG2a K isotype control PE were purchased from Thermofisher Company. BCL1 clone 5B1b cell line was supplied by Cell Bank of Pasteur Institute of Iran.
3.2. Preparation of PEG-nVCR
PEG-nVCR was prepared using the thin-film hydration technique. Span 60, cholesterol, PEG-3000, and vincristine sulfate at molar ratios of 7: 3: 1: 1 were dissolved in 15 mL of ethanol (96 %) and mixed on a magnetic stirrer at room temperature (300 rpm, 45 min). The solvent was evaporated by a rotary evaporator (Heidolph, Germany, 90 rpm, 45ºC). The resultant film was dissolved in 10 mL of phosphate-buffered saline (pH 7.4) and stirred at room temperature (300 rpm, 20 min) and followed by sonication (35 KHz, Bandelin Sonorex Digitec) for 10 min. PEG-nVCR was obtained. Also, PEGylated niosomes devoid of the drug (empty PEG-nisomes) were prepared in the same way without the addition of drugs.
3.3. Characterization of PEG-nVCR
Mean particle size, size distribution, and zeta potential of PEG-nVCR were determined by dynamic and electrophoretic light scattering using a Zetasizer instrument (Zetasizer Nano ZS, Malvern Instruments, Worcestershire, UK). The encapsulation efficiency (EE %) of PEG-nVCR formulation was determined by the ultra-centrifuge (45000 rpm, 60 min, 4ºC). Briefly, 1 mL of niosomal formulation was ultra-centrifuged, and the resultant pellet was washed twice by PBS (pH 7.4), and the amount of unentrapped drug was measured in the supernatant at 297 nm by spectrophotometer (UV-1601 PC, Shimadzu). Then, the encapsulation efficiency was calculated by the following formula using a standard curve equation:
EE % = (total VCR in niosomal batch - unentrapped VCR)/total VCR in niosomal batch × 100.
The standard curve equation was obtained by reading the optical densities (at 297 nm wavelength) of the different concentrations (serial dilutions) of the standard VCR using Excel software.
3.4. In Vitro Drug Release Study
The release rates of free VCR and PEG-nVCR were studied using the membrane diffusion technique (11). After the determination of EE% (as mentioned in the previous section), the resultant pellet was resuspended in1 ml of fresh PBS and placed in the dialysis bag (cellulose membrane, cut off 10 kD). The dialysis bag was immersed in 50 mL of PBS and placed on a magnetic stirrer (37ºC, 150 rpm) for 36 h. At predetermined interval times, 1 mL of PBS was taken and replaced with an equal volume of the fresh PBS. The optical density (OD) of each fraction was measured at 297 nm by spectrophotometry. The same procedure was performed for the Free VCR. Finally, the amount of released drug in each time point was calculated by the standard curve equation.
3.5. Stability Study
The PEG-nVCR suspension was stored in glass bottles at 4 ± 1ºC for 2 months to evaluate physicochemical stability. The stability of PEG-nVCR nanocarrier was evaluated in terms of appearance, aggregation, particle size, and zeta potential variation.
3.6. Cell Viability Study
Cell viability study was evaluated against BCL1 clone 5B1b cells by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. For this purpose, 1 × 104 cells/well were seeded RPMI-1640 medium containing 10% heat-inactivated FBS and supplemented with 100 U/mL of penicillin and 100 μg/mL streptomycin at 37ºC in a humidified atmosphere with 5% CO2 in the air. After 24 h, the medium was replaced with fresh medium containing serial dilutions of PEG-nVCR and free VCR. After 48 h of incubation, 10 µL of 5 mg/mL MTT dye solution in PBS was added to each well, and the plate was incubated for another 3 h at 37ºC to complete the reaction. Then, the medium was removed, and purple formazan crystals were resolved in 100 µL isopropanol. Optical density was measured by a microplate reader at 540 nm. The cell viability was determined by the following equation:
Cell viability = OD of test/OD of control × 100
3.7. Induction of Mouse Lymphoma Model
Animal experiments were performed according to the guidelines of the Animals Ethics Committee of Pasteur Institute of Iran (Authorization code IR.PII.REC.1395.68) and the European Communities Council Directive of 24 November 1986 (86/609/EEC). For this purpose, BCL1 clone 5B1b cells, at a number of 2.5 × 106, were suspended in 100 µL of PBS (pH 7.4) and intravenously injected to tail vein of 5 to 6-week-old female BALB/c mice (18 - 20 g, 4 groups, 10 mice per group) in three consecutive days (3 doses). A group of 8 mice (without injection of lymphoma cells) was considered as the normal group. To confirm tumor cell formation (Approximately one month after the last injection of the cells), two mice were randomly selected in each group (in total, 8 mice), and the 8 mice of the normal group were sacrificed by the cervical dislocation. Their spleen indices were calculated, and the amounts of lymphoma cells (CD5, IgM, and IgM/CD5 positive cells) were determined by the flow cytometry. A significant increase in the CD5, IgM, and IgM/CD5 positive cells, and spleen indices, compared to the normal mice, confirmed the formation of tumor cells.
3.8. In Vivo Antitumor Activity
Treatment was started after confirmation of tumor cell formation. For this purpose, each group of mice was treated by 100 µL of PEG-nVCR, free VCR (containing 2 mg of VCR/Kg body weight), PEG-niosomes devoid of VCR, and PBS every other day for three days. The weight of the animals was measured every week. A day after the last treatment, the animals were weighed and sacrificed for removing the spleens. To evaluate the antitumor efficacy, the spleen indices (spleen weight to body weight ratio) was calculated, and the number of CD5, IgM, and IgM/CD5 positive lymphocytes in spleens was determined by flow cytometry using the anti-CD5 and anti-IgM antibodies (along with IgG2a K Isotype control antibody) against the lymphoma cells which have over-expressed these markers.
3.9. Extraction and Preparation of Lymphocytes from Spleen
For the isolation of lymphocytes from spleens of the mice, the spleens were dissected and homogenized in a petri dish, and the cells were extracted into 2 mL of culture medium. Two milliliters of red blood cell lysis solution was added to the cells. The cells were briefly mixed and left for 2 min at room temperature. Then, the cells were centrifuged (1500 rpm, 5 min) after the addition of 2 mL of fetal bovine serum (FBS). After centrifugation, the cells were suspended in 1 mL of PBS and counted by the Neubauer chamber under an optical microscope. One million of the cells were mixed with anti-mouse CD5 FITC and anti-mouse IgM PE antibodies in one tube, and 1 million of the cells were mixed with IgG2a K Isotype control PE antibody in another tube. Isotype control antibodies can act as negative controls to help differentiate non-specific background signals from specific antibody signals because they have no relevant specificity to a target antigen. The tubes were placed at 4ºC for 30 min, and 2 mL of PBS was added to each one. After centrifugation (1500 rpm, 5 min), the supernatants were removed, and the cells were fixed with 1 mL of PBS containing 1% paraformaldehyde and analyzed by the flow cytometry.
3.10. Neurotoxicity Study
Neuropathy is one of the major side effects of vincristine (12, 13). The main symptoms of neuropathy generally divide into three main categories, including sensory, motor, and autonomic neuropathy (12), which mostly impact movement performance. There are several behavioral tests for assessment of motor performance, which among them the rotarod (rotating rod) is an appropriate test to evaluate motor coordination in rodents (14, 15). The effect of different formulations of VCR in improving the neurotoxicity side effects (motor incoordination) was studied on 4 groups of mice, which were previously mentioned. Before and after to injection of BCL1 clone 5B1b cells, each group of the mice was trained to stand on the rotarod for at least 3 min. They were treated with different formulations of VCR as mentioned-above. After the last treatment and before sacrificing, each group of mice was allowed to stand on rotarod and their standing times were measured.
3.11. Statistical Analysis
All results were presented as mean ± standard deviation (SD). Statistical significance of differences was determined using the one-way ANOVA and independent t-test; A P value < 0.05 was considered as statistically significant.
4. Results
4.1. Characterization of Niosomes
The results of the physical properties of niosomes are described in Table 1. They had a narrow size distribution and a mean particle size of about 220 nm. The zeta potentials of the nisomes were slightly negative and approximately -19 mV. The entrapment efficiency was obtained at about 81 %.
| Formulation Type | Parameters, mean ± SD (N = 3) | |||
|---|---|---|---|---|
| Size (nm) | Zeta Potential (mV) | PDI | EE (%) | |
| PEG-nVCR | 220.6 ± 5.7 | -18.8 ± 2.4 | 0.40 ± 0.15 | 81.2 ± 2.5 |
| Empty PEG-nisomes | 227.4 ± 4.6 | -16.5 ± 3.1 | 0.34 ± 0.12 | - |
Abbreviations: EE %, entrapment efficiency; PDI, polydispersity index
4.2. In Vitro Drug Release Study
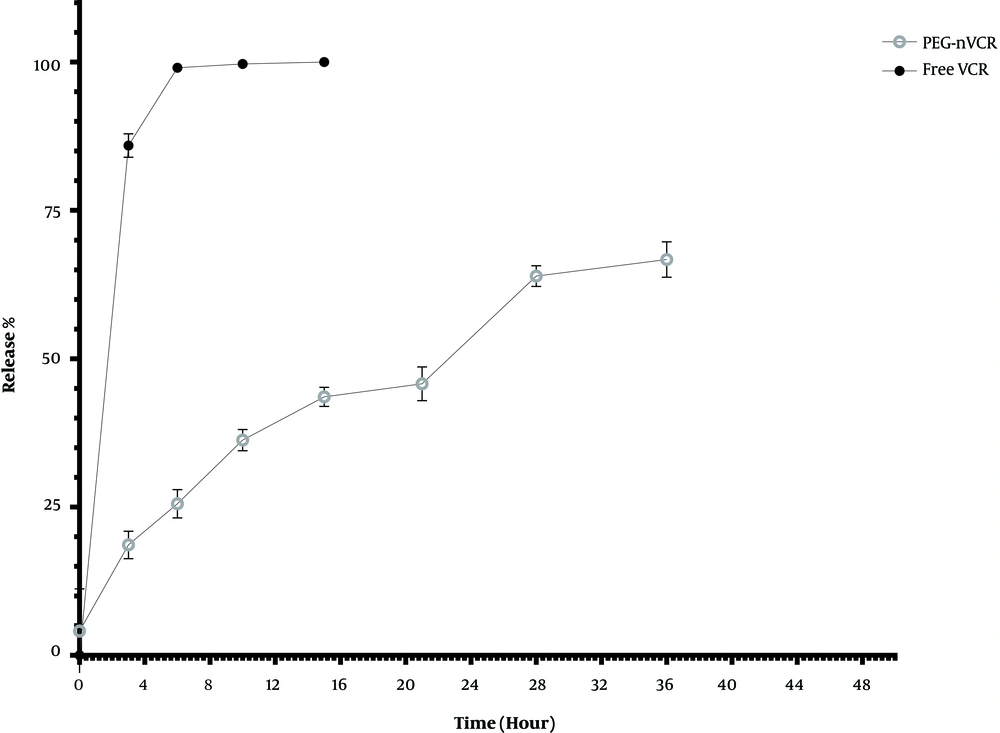
Figure 1 shows the release profile of PEG-nVCR and its free counterpart. The release of VCR from PEG-nVCR was significantly lower than that of Free VCR. Free VCR showed a rapid release pattern with a release rate of about 80% in the first 3 h. As it is depicted, about 21% of the drug was released in the first three hours from nanoparticles, and after the release of drugs from the PEG-nVCR, it took place slowly in such a way that the maximum release of VCR reached about 69% after 36 h.
4.3. Stability Study
PEG-nVCR suspension was stable for at least 2 months after production. No significant changes were observed in particle size, homogeneity, and zeta potential during the storage period. The appearance of noisomes was stable and there was no aggregation.
4.4. Cell Viability Study
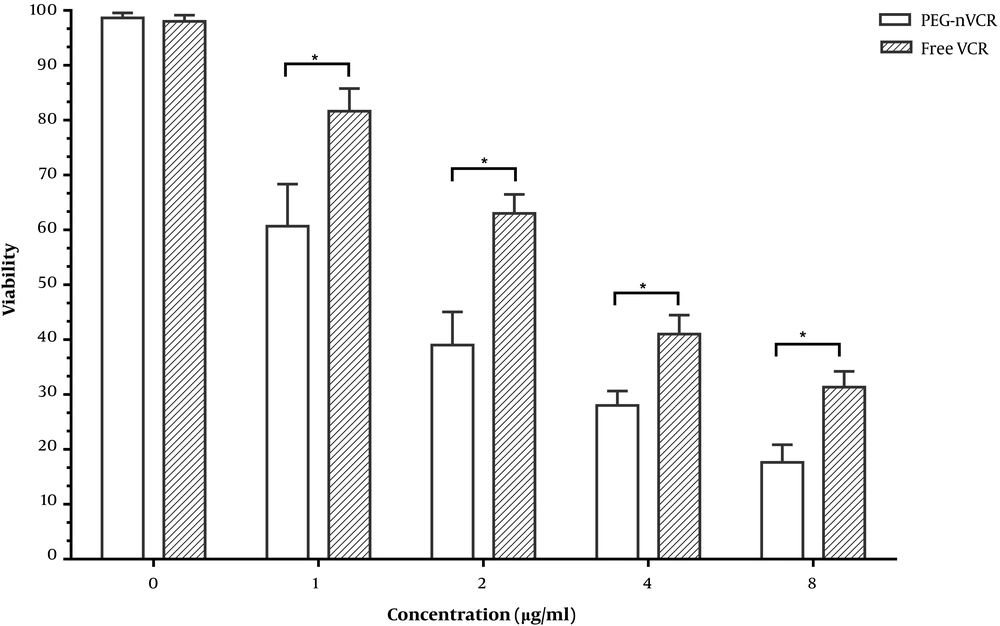
As depicted in Figure 2, the cell viability is dose-dependent. By increasing the drug concentration (from 0 to 8 µg/mL) in the form of free VCR or PEG-nVCR, the percent of the viable cells was decreased. The cytotoxicity of PEG-nVCR was significantly higher than that of free VCR.
4.5. In Vivo Antitumor Activity
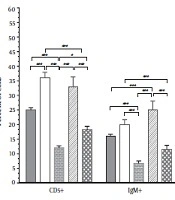
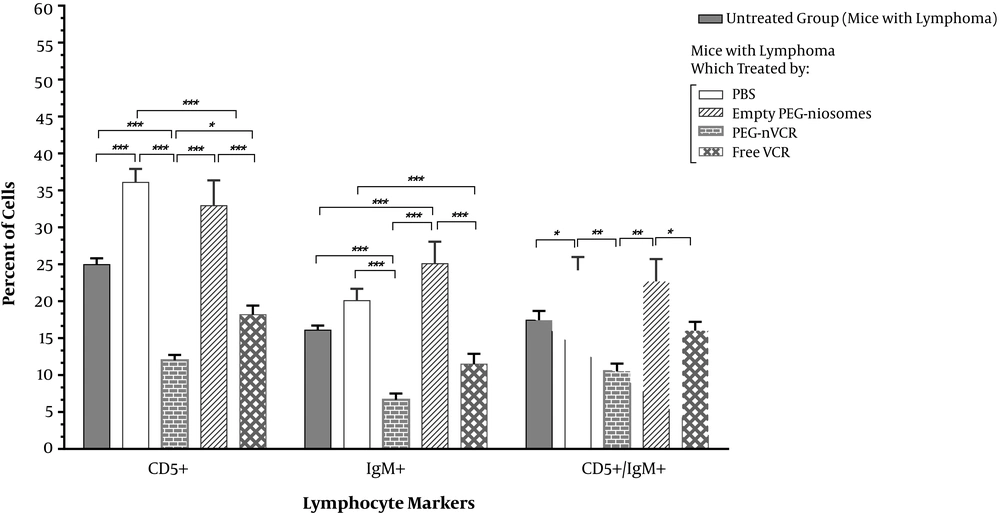
Higher expression of surface markers, such as CD5 and IgM, is reported in b-cell lymphoma (16, 17). So, the amount of CD5 and IgM positive cells is correlated to b-cell tumors. A significant reduction in lymphoma cell count (CD5, IgM, or IgM/CD5 positive cells) was obtained for the PEG-nVCR, as compared to the free VCR (Figure 3). The changes in body and spleen weight and spleen index in different groups are presented in Table 2. As shown in the table, there is a significant difference (P < 0.001) between the spleen weights and indices in normal and lymphoma cells-injected mice. Although no significant change in body and spleen weight of the mice was observed between different kinds of formulations.
| Parameter | Normal Mice | Mice with Lymphoma (Untreated) | Mice with Lymphoma Which Treated by Different Formulations | |||
|---|---|---|---|---|---|---|
| PBS | Empty PEG-Niosome | Free VCR | PEG-nVCR | |||
| BW (g) | 21.52 ± 1.02 | 21.3 ± 1.58 | 21.14 ± 0.94 | 20.68 ± 1.37 | 20.34 ± 1.81 | 20.86 ± 1.09 |
| SW (g) | 0.14 ± 0.009 | 0.17 ± 0.008 | 0.17 ± 0.007 | 0.17 ± 0.01 | 0.17 ± 0.014 | 0.16 ± 0.010 |
| SI | 0.0066 ± 0.0004 | 0.008 ± 0.0006 | 0.008 ± 0.0006 | 0.008 ± 0.0008 | 0.008 ± 0.001 | 0.008 ± 0.0005 |
Abbreviations: BW, body weight (g); SI, spleen index; SW, spleen weight (g)
aEach data represents the mean ± SD (n = 3).
Flow cytometric analysis of lymphoma cells (CD and IgM positive cells) in spleens of mice with lymphoma (untreated group) and treated groups by different formulations. Each group consists of 8 mice. Results were expressed as mean ± SD. *P value = 0.01; **P value = 0.001; ***P value = 0.0001.
4.6. Neurotoxicity Study
The results of the rotarod study showed a significant reduction in neurotoxicity symptoms in the group of mice treated by PEG-nVCR as compared to the free VCR. The group which treated by PEG-nVCR could stand on a rotating road for 98 ± 7 sec, while the time for the group which received free VCR was about 41 ± 9 sec and those of PEGylated niosomes devoid of VCR, PBS was found to be 108 ± 7 and 112 ± 11 sec, respectively. The standing time of the PEG-nVCR group on the rotating rod was about 2.5-fold longer than that of the free VCR group.
5. Discussion
With the advancement of biotechnology, much emphasis has been paid to improving the drug delivery systems. Particular attention is paid to the niosomes, as a drug delivery system. In this study, PEG-nVCR was successfully prepared and characterized. Drug release, viability study, in vivo antitumor efficacy, and neurotoxicity study were investigated. The size and zeta potential of the vesicles have a positive correlation with their stability and releasing pattern (18). The small size (220 nm) and relatively big negative charge or zeta potential (about -19 mV) of the vesicles are important factors that have positive effects on their stability, half-life, and cellular uptake (19). Zeta potential is a measure of the magnitude of the electrostatic or charge repulsion/attraction between particles and is one of the fundamental parameters known to affect stability. The negative Zeta-potential of prepared niosomes provides high physical stability to the dispersion, due to electrostatic repulsions between the particles in the suspension. The burst effect in the first 3 h of release study may be due to the release of VCR from the layers of niosomal vesicles that have not been completely entrapped. The lower releasing rate of PEG-nVCR can be related to the PEGylated niosomal barrier, which entrapped the drug and limited its diffusion and protected against environmental effects. The presence of polyethylene glycol in the composition of the niosomes induces more negative charge and thereby prevents aggregations, thus results in higher stability and lower releasing rate for PEG-nVCR (20). The hydrophilicity of PEG increases the solubility of hydrophobic drugs. It provides drugs with a greater physical and thermal stability as well as preventing or reducing aggregation of the drugs in vivo, as well as during storage, as a result of the steric hindrance and/or masking of charges provided through the formation of a “conformational cloud”. A significant reduction in lymphoma cell count (CD5-, IgM- or IgM/CD5-positive cells) was obtained for the PEG-nVCR as compared to the free VCR (Table 2). This discrepancy can be attributed to higher efficacy and lower release of drug from PEG-nVCR, as compared to the free VCR formulation (21). Slow or sustained release behavior of PEG-nVCR extends the half-life of the VCR in blood circulation and thereby enhances its antitumor or therapeutic efficacy (22). The standing time of the PEG-nVCR group on the rotating rod was about 2.5-fold longer than that of the free VCR group. This difference can be related to the sustained release behavior of PEG-nVCR that maintains a constant amount of drug in circulation and let the drug more time for action. Similar results have been previously reported by Parthasarathi et al. regarding the effectiveness of niosomes concerning the improvement of the antitumor activity of vincristine in S-180 and Ehrlich ascites in mice (23).
The viability of lymphoma cells was reversely correlated with drug concentration. By increasing the drug concentration (either in free or in noisome form), the cell viability was decreased. The PEG-nVCR showed higher cytotoxicity compared to the free drug after 48 h of incubation. The higher cytotoxicity of niosomal drugs is reported by Tila and colleagues (24). They prepared a pH-sensitive and plasma stable niosomes containing Mitoxantrone and then evaluated its cytotoxicity on OVCAR-3 and MCF-7 cell lines. The results of this study showed that the antitumor effect of the drug increases when entrapped in the niosomes.
According to the findings, the PEGylated niosomes containing VCR significantly increased the therapeutic efficacy of VCR, decreased the release of vincristine, and showed a suitable entrapment efficiency. The in vivo experiments showed that PEG-nVCR significantly increased the therapeutic efficacy of VCR and decreased the neurotoxicity symptoms in lymphoma bearing BALB/c mice. These results provide a logical starting point for using of the niosomal formulation as a promising carrier in preclinical in vivo experiments.