1. Background
Cancer is one of the chronic diseases with abundant death rate, share two major properties: abnormal cell proliferation and invasion to normal cells of the body (1). According to Bora et al. (2), after cardiovascular disease, cancer has been beholder as the main causes of death. Despite many advancements in anti-cancer therapeutic approaches in recent decades, ineffectiveness of some techniques and also drugs, encourage the scientists to find new, safe and potential alternative for overcoming the problem (3). Hence, some complementary methods were added to common regimes. Among them, identify naturally substances from herbal sources which have major role in prophylaxis and treatment of cancers is of significant interest. From ancient time, using medicinal plant for treatment of diseases like cancer, are popular. However, little information about the active substances of them are available. One of these active anti-cancer ingredients which have ancient history is cucurbitacins. Cucurbitacins are a major group of triterpenoids which were identified from Cucurbitaceae plants. They have been proposed to have many biological activities such as anti-cancer, anti-inflammatory, anti-pyretic and analgesic (4-8). E. elaterium (squirting cucumber) (Cucurbitaceae) is a succulent and perennial herb widely distributed in the Mediterranean area and Iran (9). Despite a large-scale anti-cancer researches on the fruit juice of E. elaterium (5), there is not any survey concerning the related anti-proliferative activity of the aerial parts and roots of this plant.
2. Objectives
Therefore, as a part of our duty and based on the other studies on screening programs to find different medicinal plants with biological activities especially tumor growth inhibition activity (10-18), we evaluate the anti-proliferative activity of the roots and aerial parts of this plant on MCF7 (human breast adenocarcinoma), Hep G2 (liver adenocarcinoma), SW480 (Colon adenocarcinoma) and HFFF2 (human fibroblast cell line) Cell lines as well as investigation on chemical compositions of potent extracts.
3. Methods
3.1. Plant Material
Aerial parts and rhizomes of E. elaterium were collected from Moghan-Ardabil province. The voucher number (Tbz-fph 648) of samples is entrusted to the herbarium of the Pharmacy Faculty, Tabriz University of Medical Sciences, Tabriz, Iran. Air-dried parts of the plant were sequentially extracted by Soxhlet with n-hexane (n-hex), dichloromethane (DCM) and methanol (MeOH), respectively. All extracts were condensed by rotary evaporator apart from each other.
3.2. VLC Method
For further evaluation, potent extracts were fractionated by vaccum liquid chromatography (VLC). This method was based on Targett et al. assay with some modification (10). The silica gel was first loaded into the 2/3 of the sintered VLC funnel and was left to settle by taping under gravity, then the vacuum pomp was applied till the silica gel became compact. After this process, 150 mL ethyl acetate and finally 150 mL ethyl acetate 10% (10% ethyl acetate and 90% n-hexane) passed through the silica gel, respectively. The extract which was solved in ethyl acetate 10% was washed by different concentrations of ethyl acetate-n-hexane (10, 20, 40, 60, 80, and 100%).
3.3. Cytotoxicity Assay
MCF-7 (human breast adenocarcinoma), Hep G2 (liver adenocarcinoma), SW480 (Colon adenocarcinoma) as cancerous and HFFF2 (human fibroblast cell line) as normal cell lines, were purchased from Pasture Institute, Tehran, Iran and was cultured in a humidified atmosphere at 5% CO2, in Roswell Park Memorial Institute Media (RPMI-1640), supplemented with 10% Fetal Bovine Serum (FBS). Cells were cultivated in 96-well plates at a density of 15 × 106 cells per well in 100 µL of culture medium for 24 h. Extracts were solubilized in DMSO, then diluted in culture media for use. The final concentration of DMSO in the culture medium was maintained at 1% (v/v) to avoid solvent toxicity. 100 μL of different concentrations of the samples (10, 50, 100, 300, 400, 600, 800 µg/mL) were applied to the wells of a 96-well plate containing the confluent cell monolayer in duplicate. DMSO was tested as solvent control, while Methotrexate was used as a reference standard. After 48 h incubation, Cell viability was assessed by MTT assay and the IC50 values was calculated and compared with that of the control using OD values of viable cells (1).
3.4. GC-MS and GC-FID Analyses
GC/MS analysis of the potent extracts and fractions of E. elaterium was carried out on a Shimadzu GCMS-QP5050A gas chromatograph-mass spectrometer (GC-MS) fitted with a fused methyl silicon DB-1 column (60 m × 0.25 mm i.d., 0.25 μm film thickness). Carrier gas was helium with a flow rate of 1.3. The column temperature was programmed at 100ºC for 2 min and then increased to 300ºC at a rate of 4ºC/min increase, and finally kept constant at 300ºC for 15 min. The injector temperature was 270ºC and split ratio was set up at 1:19. The injection volume was 1 mL. The mass operating parameters were obtained at the following conditions: ionization potential 70 eV; ion source temperature 260°C; quadrupole temperature 100°C; solvent delay 2.0 min; resolution 2000 amu/s and scan range 30-600 amu; EM voltage 3000 volts.
Components of the essential oil were identified by comparison of their mass spectra with those for standard compounds, and computer matching with the NIST 107, NIST 21, NIST 69 and Wiley 229 mass spectral database, as well as by comparison of the fragmentation patterns of the mass spectra with those reported in the literature (19).
For quantitation (area %), the GC analysis was also performed using an Agilent 6890 gas chromatograph equipped with a FID detector. The FID detector temperature was 300ºC.
3.5. Phytochemical Analysis
3.5.1. Steroids, Triterpenoids and Glycosides Tests
Libermann-Buchard test: little amount of acetic anhydride were added to the different samples and mixed together. Subsequently, by adding the concentrated H2SO4 slowly, brown ring at the gap of two layers was observed through one hour. The color changes in up and down layer were used to distinguish the steroids from triterpenoids. The Libermann-Buchard, Kedd’s and Keller-Killiani tests for glycosides (10).
3.5.2. Tests for Alkaloids
According to the Dragendorff’s and Hager’s tests
3.5.3. Test for Tannins
Dark green color was appeared in samples which have tannins, by adding the 5% ferric chloride.
3.5.4. Test for Flavonoids
Shinoda test: Amount of HCl 37% with all test samples was mixed, Magnesium ribbon was added for increasing the speed of color changes to red.
3.5.5. Test for Carbohydrate
Benedict’s test was used according to Heshmati Afshar et al. (11) method.
4. Results and Discussion
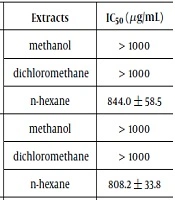
The ordinary treatment for malignancy is inefficient because of deleterious adverse effects. Resistance against the anti-proliferative substances has increased, and subsequently, it has been predicted that the future of chemotherapy will worsen. Hence, the scientist’s concentration has shifted toward herbal products. Serious anti-cancer investigations into the potency and efficacy of natural products (fruits, vegetables, plants, and spices) have been conducted with positive results (20). In the first step of the current study, the anti-proliferative capability of different extracts from the rhizome and aerial parts of E. elaterium on a variety of solid tumor strains such as HepG2, SW480, and MCF7 as cancerous cell lines and HFFF2 as normal cells was widely measured. From Table 1, it can be inferred that sensitivity to the growth inhibition potency of all extracts varied adequately between cell lines. Subsequently, the findings showed that among the six extracts, the n-hexane extract of the aerial part of the squirting cucumber exhibited the highest inhibition ability (lowest IC50), particularly toward the MCF7 cell lines in in vitro experiments (compared with the positive control, methotrexate). Other extracts demonstrated an inadequate capability of slowing down all cancerous cell growth. However, n-hexane extract showed minimum inhibitory effect on normal cell lines (HFFF2). For this reason, the n-hexane extract of the aerial parts is a candidate for more investigation. In the next step, the anti-proliferative potency of the VLC fractions of the potent extract was assessed, and the results are presented in Table 2. The 100% fraction had a minimum survival rate among all the fractions against cancerous cells and also minimum toxicity on normal cells. Unbelievably, the 80% VLC fraction not only indicated a growth effect on the cancerous cell line, but also illustrated a strong anti-proliferative effect on normal cells. Our results in some cases are inconsistent with others findings, which represent the inhibition of main constituents in the juice of the squirting cucumber on different cancerous cells with diverse methods (21-23). As an exemplification, the anti-proliferative activity of cucurbitacins as main compounds in the fruit juice of E. ellaterium on a broad range of tumor cells (HepG2, MCF7) was investigated in recent studies (24, 25). In all investigations, the findings illustrated that cucurbitacins as triterpenoid substances were responsible for the anti-tumor activity of this plant. Furthermore, they showed that these secondary metabolites have chemo-preventive activity through their effect on microtubules by means of the apoptosis pathway. The current findings are compatible with the popular use of this plant in folk medicine to treat sinusitis and cancers in Moghan and other cities (5). Even though anti-proliferative evaluations of the juice and seeds of E. elaterium have been conducted, no cytotoxic assessment on the aerial parts and roots of this plant has been done. In our research, the cytotoxic activity of n-hexane extract prompted researchers to survey and identify vigorous active functional groups. Preliminary phytochemical analysis of n-hexane extract and 100% VLC fraction proved that these samples are rich in steroids (Table 3). Hence, it may be concluded that the potency of cancer cell inhibition of this extract is may be due to the presence of these ingredients. Other published investigations have reported the potency of steroids as anti-cancer agents (26-28). Moreover, GC-MS inspection of n-hexane extract (Table 4) reveled broad ranges of compounds such as n-Hentriacontane, neophytadien, phytol and alpha-tochpherol with 73.97, 4.89, 3.59 and 3.51 % contents quantitavely, respectively. Furthermore, 100% VLC fraction illustrated (Table 5) discrete groups of secondary organic metabolites like, Loliolide, Thymol, carvacrol, Neophytadine, Limonene dioxide as the main components with high relative area percentage, respectively. In previous different studies the growth inhibition effects of different types of steroids, fatty acids, terpenoids, and hydrocarbons, on various cancer cell lines have been also demonstrated (1, 10-13, 18, 29). For instance, Deepak et al. examined the phytochemical profile and anti-proliferative potency of plant. They identified n-hentriacontane as a potent growth inhibiting agent (30). Other study has investigated the anti-proliferative activity of neophytadiene as an important volatile ingredient which has been showed this compound inhibits tumor cells in a time and dose dependent manner (31). Constantino and Olivera et al. confirmed that vitamin E and its derivatives inhibit cancerous cells and also increase the efficacy of neoplastic drugs such as doxorubicin (32). Moreover, phytol as a diterpenoid compound inhibits cancer cells by inducing apoptosis (33). Other different studies proved the cytotoxic activity of volatile components presents in the 100% fraction (34-36). One report on the selective anti-proliferative performance of loliolide, clearly showed that loliolide inhibited the growth of nasopharynx carcinoma cells and decrease the level of P21 protein and reactive oxygen species, which led to destruction of cancer cells and delay in aging of fibroblast cell lines (normal cells) (34). Various other investigations have also confirmed the anti-proliferative activity of monoterpenes (such as thymol, carvacrol, and limonene) by DNA fragmentation, which decrease the mitochondrial membrane potential and activates the caspase activity which causes apoptosis (36). Although previous studies have attributed the anti-proliferative activity of fruits and seeds of E. elaterium to the presence of cucurbitacins (triterpenoids) (4-8, 23), the current study showed that monoterpenoids play an important role in this effect. It seems that the anti-proliferative activity of the aerial and rhizome parts) is low compared to that of the fruit juice which was conducted in previous publish papers. It may be due to the presence and accumulation of triterpenoids (like cucurbitacins) as potent cytotoxic agents in fruit juice.
| Cell Line | Samples | Extracts | IC50 (µg/mL) |
|---|---|---|---|
| HepG2 | Rhizome | Methanol | > 1000 |
| Dichloromethane | > 1000 | ||
| n-hexane | 844.0 ± 58.5 | ||
| Aerial parts | methanol | > 1000 | |
| Dichloromethane | > 1000 | ||
| n-hexane | 808.2 ± 33.8 | ||
| MCF7 | Rhizome | Methanol | 393.3 ± 6.04 |
| Dichloromethane | 520.6±39.5 | ||
| n-hexane | 321.1 ± 40.2 | ||
| Aerial parts | Methanol | > 1000 | |
| Dichloromethane | 516.3 ± 39.4 | ||
| n-hexane | 264.3 ± 5.2 | ||
| SW480 | Rhizome | Methanol | > 1000 |
| Dichloromethane | > 1000 | ||
| n-hexane | 823.4±101.3 | ||
| Aerial parts | Methanol | > 1000 | |
| Dichloromethane | > 1000 | ||
| n-hexane | > 1000 |
| Fractions (% Ethyl Acetate in n-hex) | IC50 (MCF7) µg.mL-1 | % Growth in 500 µg.mL-1 | IC50 (HFFF2) µg.mL-1 | % Growth in 500 µg.mL-1 |
|---|---|---|---|---|
| 10% | Stimulation effect | 106.7 | Stimulation effect | 102.0 |
| 20% | > 1000 | 83.4 | Stimulation effect | 100.7 |
| 40% | Stimulation effect | 102.4 | Stimulation effect | 101.9 |
| 60% | > 1000 | 90.5 | > 1000 | 93.7 |
| 80% | > 1000 | 61.9 | 431.1 ± 26.8 | 41.2 |
| 100% | 351.2 ± 5.5 | 38.6 | 948.5 ± 83.8 | 56.4 |
Abbreviations: n-hex, n-hexane; VLC, vaccum liquid chromatography.
| Phytochemical Tests | Samples (1) | ||
|---|---|---|---|
| n-hex | 100% VLC Fraction | ||
| Alkaloids | Dragendorff’s test | - | - |
| Hager’s test | - | - | |
| Glycosides | Kedd | ++ | ++ |
| Keller-Killiani | - | - | |
| Tannins | Ferric chloride test | - | - |
| Flavonoids | Shinoda test | - | - |
| Sterol | Libermann-Buchard test | +++ | +++ |
| Terpenoid | Libermann-Buchard test | ++ | ++ |
| Carbohydrate | Benedict’s test | - | - |
Abbreviations: n-hex, n-hexane; VLC, vaccum liquid chromatography.
| No. | Compound | Rt. | Area, % | Method |
|---|---|---|---|---|
| 1 | Loliolide | 29.601 | 1.66 | GC-MS |
| 2 | 1-Allyl-1-but-3-enyl-1-silacyclobutane | 29.777 | 1.51 | GC-MS |
| 3 | Neophytadiene | 33.088 | 4.89 | GC-MS |
| 4 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 34.217 | 1.16 | GC-MS |
| 5 | Hexadecanoic acid | 35.788 | 3.36 | GC-MS |
| 6 | Linolenic acid methyl ester | 39.253 | 1.04 | GC-MS |
| 7 | Phytol | 39.848 | 3.59 | GC-MS |
| 8 | Propylhexedrine | 47.335 | 1.29 | GC-MS |
| 9 | n-Hentriacontane | 52.525 | 73.97 | GC-MS |
| 10 | n-Pentacosane | 59.205 | 1.75 | GC-MS |
| 11 | Alpha-Tochpherol | 62.589 | 3.51 | GC-MS |
Abbreviation: n-hex, n-hexane.
aCompounds listed in order of elution from a DB-1 column
bTotal identified = 97.73%; non-terpennoides = 94.14%; hydrocarbon = 86.23%; fatty acid and derivatives = 7.91%; terpenoids = 3.59%.
| No. | Compound | Rt. | Area, % | Method |
|---|---|---|---|---|
| 1 | Heptane, 3,4-dimethyl | 5.125 | 3.25 | GC-MS |
| 2 | Thymol | 20.072 | 12.05 | GC-MS |
| 3 | Carvacrol | 20.37 | 6.09 | GC-MS |
| 4 | 5-Eocosene(E) | 28.833 | 6.51 | GC-MS |
| 5 | Limonene dioxide | 30.164 | 7.61 | GC-MS |
| 6 | Loliolide | 32.008 | 17.93 | GC-MS |
| 7 | Neophytadine | 34.707 | 7.67 | GC-MS |
| 8 | 7,10-Pentadecadiynoic acid | 41.044 | 4 | GC-MS |
Abbreviation: n-hex, n-hexane.
aCompounds listed in order of elution from a DB-1 column
bTotal identified = 65.11%; non-terpennoides = 39.36%; hydrocarbon = 35.36%; fatty acid = 4%; terpenoides = 25.75%; oxygenated monoterpene = 25.75%
4.1. Conclusion
In general, results of this survey indicate that, the inhibitory activity of n-hexane extract and its 100% VLC fraction of aerial parts on the MCF7 cell line might be because of presence of the various anti-proliferative agents (such as steroids, monoterpenes, etc.), It is suggested that further analysis should be done to obtain the pure compounds of the 100% VLC fraction of n-hexane extract from the aerial parts of E. elaterium and evaluate the growth inhibition potency of pure compounds against cancerous cells.