1. Background
As it is known, aging is due to neuroendocrine and immune dysregulation, accumulation of damaging effects of free radical processes, decrease in adaptive capacity of the body, as well as a number of systemic involutional changes (1-3). Dynamics of multi-level age-related changes, apparently, also depends on the characteristics of the aqueous medium of the body, in particular its isotopic composition. Thus, the question of the effect of deuterium depleted water (DDW) on age-related changes presents a particular interest both in connection with the specifics of the water isotopic composition in some geographical regions (for instance in high mountain areas) that feature a sufficiently high number of centenarians, and with the appearance of technical possibilities for a wider use of artificially produced water with modified isotopic composition in the drinking ration (4).
It is known that relatively small fluctuations in the deuterium contents in the body cause changes in physicochemical properties of intracellular water (5, 6), and have a marked impact on the dynamics of biochemical, cellular, tissue, and systemic regulatory processes (7).
It is shown that the antioxidant (8, 9) and antitoxic effect of DDW (10), as well as positive effects of these nutritional factors on the state of various protective systems of the body (11), activities decline with age. In addition, there is extensive information about the impact of DDW on the growth of healthy and tumor cells, as well as its systemic antitumor activity (12, 13). According to a certain similarity of numerous biological processes observed both in aging and oncogenesis (14, 15), it can be assumed that DDW can have geroprotector properties.
The validity of this assumption is supported by the information about the possible negative impact of deuterium on DNA repair processes (16), and the evidence of the positive influence on the people’s life expectancy and health status, exerted by the water from the Baikal Lake (the deuterium concentration in which is 137 ppm (17, 18)), and the water generated at thawing of mountain glaciers (deuterium concentration -133.0 ppm (19)) or snow falling in the Arctic zone (deuterium concentration -124.6 ppm (20)).
At the same time, even with a very large number of studies that indirectly indicate the existence of geroprotector properties of DDW, no direct evidence could be found in the literature regarding the possibility to restore the physiological functions of mammals lost with age using this factor.
The current study aimed at investigating the influence of DDW on the sexual (estrous) cycle, adaptation status, and locomotor activity in white outbred presenile female rats.
2. Methods
The current study was approved by the Bioethics Committee of the Oncology Rostov Research Institute of Russian Health Ministry, protocol No. 3 from 14.03.2014. The current study respected the international rules of human treatment of experimental animals. The experiments were conducted on 37 white outbred female rats: 10 young animals (age: 8 - 10 months, weight: 210 ± 12 g) and 27 presenile rats (age 20 - 22 months, weight: 291 ± 32 g), that were divided into group 1 (n = 13) and group 2 (n = 14). In contrast to the rats in group 2 and group young, the animals in group 1 received DDW with a deuterium content of 46 ± 2 ppm in a volume of 25 to 30 mL per day per head (rat) for three weeks. According to NMR (nuclear magnetic resonance) spectroscopy, DDW contained about three times less deuterium than the natural water of the region where these rats lived (150 ± 2 ppm). Deuterium depleted water was produced on a plant designed in the Kuban State University, by the electrolytic decomposition method. Mineralization of the produced water was affected by adding salts, to achieve a physiologically high-grade mineral composition identical to that of water with a deuterium content of 46 ppm and 150 ppm (314 - 382 mg/L). In addition, the female rats in all the studied groups received standard concentrated compound feed (State Standard GOST R 50258-92, Russian Federation) ad libitum, with the same isotopic composition in terms of deuterium (142.3 ppm).
As the indicators of the animals’ condition, the duration of the estrous cycle phases, the dynamics of the nature of general nonspecific anti-stress adaptation reactions of the body, the skin autoflora state, and the deuterium content in the blood plasma and some visceral organs (liver, kidney, and heart) were studied.
It is well known that the cell composition of vaginal swabs of female rats in the reproductive age is determined by the state of hormonal function of their ovaries and has characteristic features that correspond to the sexual cycle phases - prooestrus, oestrus, meta oestrus, and dioestrus (21). Thus, based on the results of microscopic examination of such vaginal swabs, it is possible to assess the changes in the estrous cycle and, respectively, the severity of disorders in the neuroendocrine regulation of reproductive processes in presenile female rats. The cellular composition of vaginal swabs of female rats in the studied groups was examined on a daily basis using a Leica DM LS2 microscope, twice a day (morning and evening). According to the results of a cytological analysis, the duration and the sequence of estrous cycle phases were identified, which allowed for assessing the periodicity of hormonal changes in the ovaries of the animals in the studied groups.
To characterize the adaptation status that determines the body resistance to damaging factors of different nature, the skin microbial resistance was assessed. When studying the bacterial prints from the animal's tails, meat-peptone agar with 1.5% bromothymol blue alcohol solution and 1% mannitol solution was used. The differentiation between the colonies of pathogenic and non-pathogenic bacteria was affected based on the change in the bromothymol blue color due to mannitol fermentation by pathogenic forms of staphylococci. Herewith, the colonies of non-pathogenic bacteria were green, and the colonies of pathogenic staphylococci were yellow. At the end of the experiment, the animals of the studied groups were euthanized by decapitation under ether narcosis, whereupon samples were taken of blood and visceral organs (liver, kidney, and heart).
The deuterium concentration in plasma was determined on NMR spectrometer JEOL JNM-ECA 400MHz (22). To determine the isotopic composition of lyophilized (in a freeze dryer LS-1000, Prointex, RU) organs of laboratory animals, a mass-spectrometer DELTAplus H/Device (Finnigan, Germany) was used (23). Statistical processing of the obtained data were performed using the variation statistics methods: the average values in the obtained samples (M) and the root mean square deviation (σ) were calculated and considered reliable; the difference between the groups was determined using the non-parametric Mann-Whitney U test where P < 0.05.
3. Results
Due to the need to study in depth the methods of exerting the DDW systemic effect on living organisms, the question of interest was whether there were any changes in the deuterium content in the experimental animals’ plasma and tissue. Table 1 provides data of NMR spectroscopy of plasma and visceral organs (liver, kidney, and heart) of presenile female rats of the tested groups, obtained before the experiment and five weeks after starting the same.
a Different from the similar indicators in group 2; P < 0.05.
The data presented in Table 1 indicate the existence of a D/H gradient between blood and organs under normal conditions: CD visceral organs < CD plasma. DDW consumption by animals in group 1 resulted in considerable reduction in the deuterium content in their bodies. The most significant changes were reported in the plasma of group 1 rats that their values of the heavy hydrogen isotopes reduced by 33%, compared with those of group 2 and the young group animals. In the organs of group 1 animals, there was also a significant, although less pronounced, reduction in the deuterium content, compared with the values in the rats of the other two groups (liver -7.2%, kidney -9.7%, and heart -7.3%). The reported changes led to the formation a new D/H isotopic gradient in the body of the test group animal (CD visceral organs > CD plasma) the opposite of the physiological D/H gradient ( CD visceral organs < CD plasma).
Table 2 presents the data on the duration of the estrous cycle phases in female rats of the test groups at the end of the experiment, including after receiving DDW for five weeks by the presenile animals in group 1. As shown in the the the estrous cycle of presenile animals in group 2, that received plain water, differed quite markedly from that of the cycle of young animals and was characterized by a significant shortening of the prooestrus and oestrus phases (3.2 and 1.7 times, respectively), with a significant extension of the meta oestrus (3.4 times) and dioestrus (1.6 times) phases. The above changes approximated the duration of different cycle phases, as well as their ratio to the corresponding values in young rats (Table 2).
a P, O, M, and D are the estrous cycle phases: prooestrus, oestrus and meta oestrus, dioestrus, respectively; ≈P:O:M:D is the ratio between the estrous cycle phases (rounded to integer values).
bA, Different from the figures in female rats in group 2 (P < 0.05); B, Different from the figures in young animals (P < 0.05).
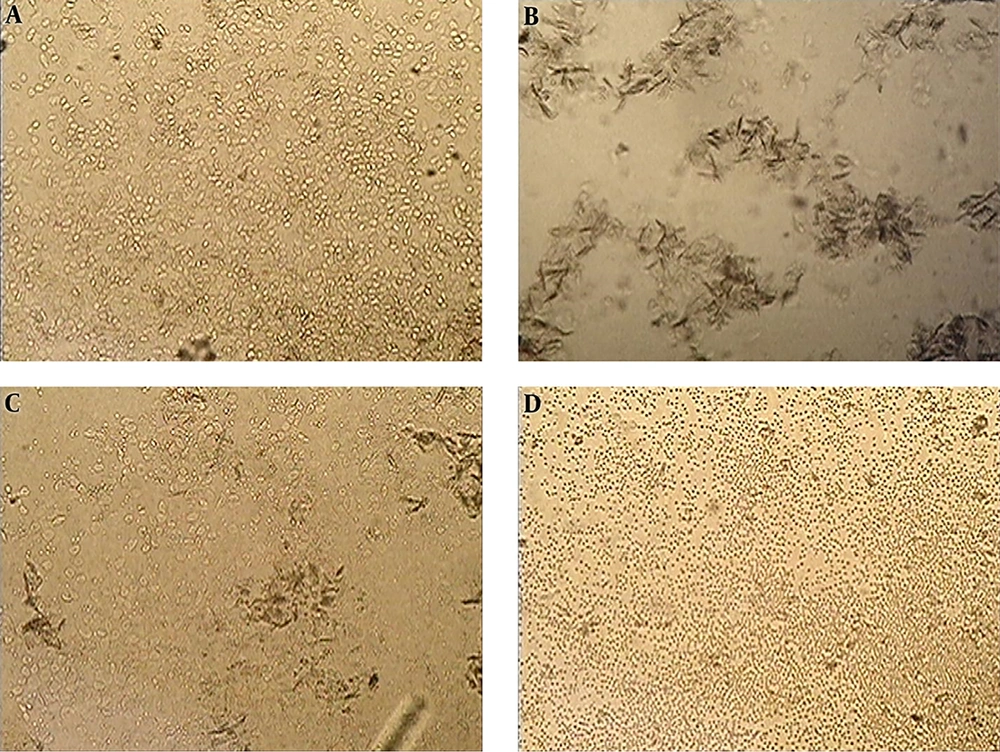
Figure 1 shows the samples of vaginal swaps obtained from presenile female rats receiving DDW, which their compositions corresponded to cellular standards of the estrous cycle phases. In the prooestrus phases, epithelial cells predominated, in the oestrus phase and scales; in the meta-oestrus phases, a mixed cells composition was recorded (horny scales, epithelial cells, leukocytes), and in dioestrus phase, leukocytes (Figure 1).
The restoration of neuroendocrine regulation due to the DDW effect explains the presence of visually distinct signs of rejuvenation of animals in group 1. Thus, the females receiving DDW showed changes in the coat condition: replacement of yellow over-hair with white, soft and shiny coat, as well as a notable increase in their locomotor activity.
The effect of DDW on the changes in the adaptation status of test group animals was assessed in terms of the skin autoflora composition (Table 3).
a P < 0.01 compared with the figures in group 2.
In case of DDW influence, yellow colonies of pathogenic staphylococci were not observed in the bacterial prints of the animals’ tail skin, whereas in the rats of group 2, such colonies significantly prevailed over the green colonies of non-pathogenic forms of staphylococci.
4. Discussion
As the animals in the experimental conditions received food with an unchanged isotopic composition, the reported changes could be due to the DDW consumption only. Herewith, the reduced content in plasma results in its reduced content in visceral organs as well, apparently, due to replacement of deuterium with protium in hydroxyl (-OH) and thiol (-SH) groups, as well as in the primary and secondary amino groups (-NH2,=NH). Such replacement of deuterium by protium in active and allosteric centers of enzymes may change the speed of catalytic reactions due to the reduction in the activation energy of transition states of the enzyme-substrate complex (24-27), which may serve as a basis to develop the metabolic adaptation of the systemic level. With replacement of deuterium with protium, there is a change in the energy of covalent chemical bonds, and the intermolecular interaction forces (for example, due to the change in the energy of hydrogen bonds) between individual molecules (28).
The formation of a new D/H isotopic gradient, opposite to the physiological gradient, may result in the increased activity of humoral and cellular protective systems, leading to a non-specific phenomenon of increase in the body resistance due to the preconditioning (29), in which protective mechanisms are potentiated at the cellular level.
At the same time, with a decrease in the isotopic D/H ratio in plasma and visceral organs, the female rats in group 1 showed some changes in their appearance and the incidence of various ARs, being characterized by shifts in the quantitative composition of peripheral blood formed elements, as well as some differences in the duration of the estrous cycle phases (according to the cellular composition of vaginal swabs), and the quantitative and qualitative composition of the skin autoflora.
Consumption of DDW for five weeks resulted in some well-marked changes in the estrous cycle, testifying to a partial restoration of neuroendocrine regulation of the reproductive function in presenile female rats in group 1. Thus, by the end of the experiment, the meta-oestrus and dioestrus phases in these animals significantly reduced (1.5 and 1.8 times, respectively), compared with those of the rats in group 2, and the duration of the prooestrus phases increased more than two times, which is characterized, as is known, by sharp shifts in the hormone secretion of the hypothalamic-pituitary-gonadal axis that acts as a trigger for the formation of the regular estrous cycle (30).
The results of the study on the effect of DDW on the animals’ adaptive status showed the activation of the skin bactericidal power mechanisms.
Thus, the obtained results demonstrated the correction, using DDW, of several processes disturbed by the development of age-related changes in presenile female rats. Furthermore, a partial restoration of some functions lost with age occurred against the background of a well-pronounced action of DDW. It was previously observed that the restoration of the dioestrus and prooestrus phases duration complement the idea of possible serotonergic mechanisms of the positive effect by reducing the deuterium concentration in the body.
4.1. Conclusion
The results of the current study are experimental evidence of the geroprotector effect of DDW on mammals manifested in the changed duration of individual phases of the estrous cycle (dioestrus, prooestrus, and meta-oestrus) and the ratio between the duration of the sexual cycle phases in general in white outbred presenile female rats and the approximation of these indicators to the similar figures in young animals, as well as in some marked signs of the coat state improvement, which becomes whiter, softer, and shinier. This is characterized by minor differences between the figures in presenile female rats receiving DDW and the similar values in young animal, in contrast to significant differences with similar data (the duration and the ratio of individual states of the estrous cycle, the changes in the cell composition of vaginal swaps in terms of norm types) that characterize the estrous cycle in presenile animals receiving water with a deuterium concentration of 150 ppm. Furthermore, the results of the current study showed a significant increase in the skin bactericidal power, as well as well-pronounced signs of the coat condition improvement. The reported systemic changes, testifying to the restoration of neuroendocrine regulation disturbed in presenile animals, were obviously due to the decreased deuterium content in blood and visceral organs (liver, kidney, and heart), resulting in the formation of a new D/H isotopic gradient (CD visceral organs > CD plasma) - opposite to the physiological D/H gradient ( CD visceral organs < CD plasma). The efficiency of DDW and the ease of using this nutritional factor make it possible to consider it as a prospective means of holistic geriatric care in the presenile period of ontogenesis.