1. Background
Hypertension is a major public health problem and a primary cause of death in the developed countries and it is growing progressively (1). Alternative Medicine including nutrition and herbal therapy play a critical role in prevention of hypertension. Consequently, continuous search to find food ingredients that have a positive effect on blood pressure (BP) is ongoing (2). Prangos ferulacea (L.) has therapeutic effects and has been used in traditional medicine for treatment of different diseases (3). In addition, P. ferulacea is a rich source of antioxidants as its antioxidant activity is higher than α-tocopherol (vitamin E) (4, 5). The extract of P. ferulacea reduces spontaneously and oxytocin-induced contraction in uterine smooth muscle (6). The acetone extract of P. ferulacea also inhibits ileum contraction, while the extract at lower concentration potentiates the contraction. Osthole is a main coumarin of P. ferulacea that relaxes ileum contraction (7). Moreover osthole was isolated from the dried fruits of Cnidium monnieri has hypotensive effect in stroke-prone spontaneously hypertensive rats (8).
Nitric oxide (NO) is a major factor in regulating BP, as any disorder in production or action of NO increases BP. Chronic prescription of L-NAME (NO synthase inhibitor) in laboratory animals increases BP and levels of lipid peroxidation products. This model of hypertension is to some extent similar to essential hypertension in humans (9) that was used in this study.
2. Objectives
Altogether, only two studies have examined the effects of osthole and d-limonene on BP, which were prepared from Cnidium monnieri and Sigma Chemical Co., respectively. A lot of effective compounds have been reported in the extract of P. ferulacea, including osthole and d-limonene, but the effects of P. ferulacea extract on BP have not yet been studied. So its acute effects and mechanism of action on BP of normal rats have been investigated in this study. Also chronic oral effects of P. ferulacea on BP and anxiety behavior in normal and hypertensive rats have been considered.
3. Methods
The protocol of the experiments carried out in this study is in accordance with the instructions for working with animals in Semnan University of Medical Sciences, Iran (license number: IR.SEMUMS.REC.1396.244) and National Institutes of Health Guide for Care and Use of Laboratory Animals. Male Wistar rats have been prepared from the animal house of Semnan University of Medical Sciences, Semnan, Iran. Animals in individual cages were placed in a 12 hour light / dark cycle at 22 - 24°C and had free access to food and water.
The drugs and chemicals used in this study including NG-nitro-L-arginine methyl ester (L-NAME), atropine, and indomethacin were obtained from Sigma-Aldrich; thiopental sodium was obtained from Kwality Pharmaceutical Pvt. Ltd. India.
3.1. Plant Extract Preparation
The Prangos ferulacea plant was prepared in the spring of 2017 from the altitudes around Shahmirzad city in Semnan province, Iran, and was verified by botanical experts in the Semnan Agricultural Development, Education and Research Organization. Aerial part of the plant was dried in shadow, ground and its hydroalcoholic extract prepared by soxhlet apparatus via repeated distillation methods during 12 hours. The extract was dried at 40°C and stored in a refrigerator. The extract yield was about 10%. During the experiments, P. ferulacea dosages were prepared daily by dilution of the extract by saline.
3.2. Experimental Procedure
Eighty male Wistar rats used in this study that were randomly divided into 12 experimental groups (n = 6 in chronic groups and n = 7 in other groups). In all groups, animals were anesthetized with thiopental sodium (80 mg/kg, ip) and placed on a rat temperature unit (Narco Bio-system, USA) to maintain a rectal temperature of 36.5 ± 0.5 °C. The femoral artery and vein were cannulated for mean arterial blood pressure (MABP) recording (pressure transducer P-1000B, Narco Bio-system, USA) and intravenous injection, respectively as previously reported (10). Briefly, after ensuring a deep anesthetic, the animal is placed on the control unit of the temperature, shaving the groin, and cutting the skin with scissors. Under the surgical microscope, femoral artery and vein dissected from each other and the nerve. The proximal part of the vessel is temporarily closed by a clamp and its distal head by surgical thread. A transversal incision is created on the surface of the vessel. The tip of the canola P 50 containing heparinized saline 10 I.U/mL is then inserted into the artery and fixed by surgical thread.
To assess the acute effects of hydroalcoholic extract of P. ferulacea on normal BP and heart rate, 28 rats (250 - 300 g) were divided into 4 experimental groups. The control and treatment groups received vehicle (saline) or P. ferulacea, respectively at doses of 12.5, 25, or 50 mg/kg intravenously (iv). To evaluate the probable mechanism through which P. ferulacea affected BP, another 28 normal rats were divided into 4 groups that received L-NAME (4 mg/kg), atropine (1 mg/kg), indomethacin(5 mg/kg), or vehicle (saline) intraperitoneally, 10 minutes before they received the P. ferulacea (50 mg/kg, iv).
Chronic nutritional effects of P. ferulacea on BP were assessed in hypertensive and normotensive rats. Twenty four male rats (200 - 220 g) were divided to four groups: (1) L-NAME + saline, (2) L-NAME + P. ferulacea (500 mg/kg), (3) water + saline, (4) water + P. ferulacea (500 mg/kg). For induction of hypertension, L-NAME (40 mg/kg/day) was administered into drinking water 4 weeks (10). At the same time, P. ferulacea extract (500 mg/kg/day) or its vehicle was prescribed by oral gavages. The rats were evaluated for anxiety behaviors and then anesthetized and BP was recorded.
3.3. Anxiety Measurement in the Elevated Plus Maze Test (EPM)
To evaluate the anxiety behavior of the rats in chronic groups, the animal was placed in the center of the elevated plus maze in 27th day of experiment. The animal was given 5 minutes to search the device. Time spent in, and entries into the open and closed arms were measured during each 5 min test. The apparatus was cleaned after each trial with water (11).
3.4. Statistical Analysis
The results of this study are presented as the mean ± SEM; a p value of P < 0.05 was accepted as statistically significant difference. The Paired t-test was used for within-group comparisons, and one-way analysis of variance (ANOVA) was used between-group comparisons, which was followed by the Tukey’s test. When the data failed the normality test, ANOVA on ranks (Kruskal - Wallis) were used, followed by Dunn’s method for multiple comparisons (SigmaStat 3.0).
4. Results
4.1. Systemic Effect of P. ferulacea on BP of Normal Rats and Its Mechanism of Action
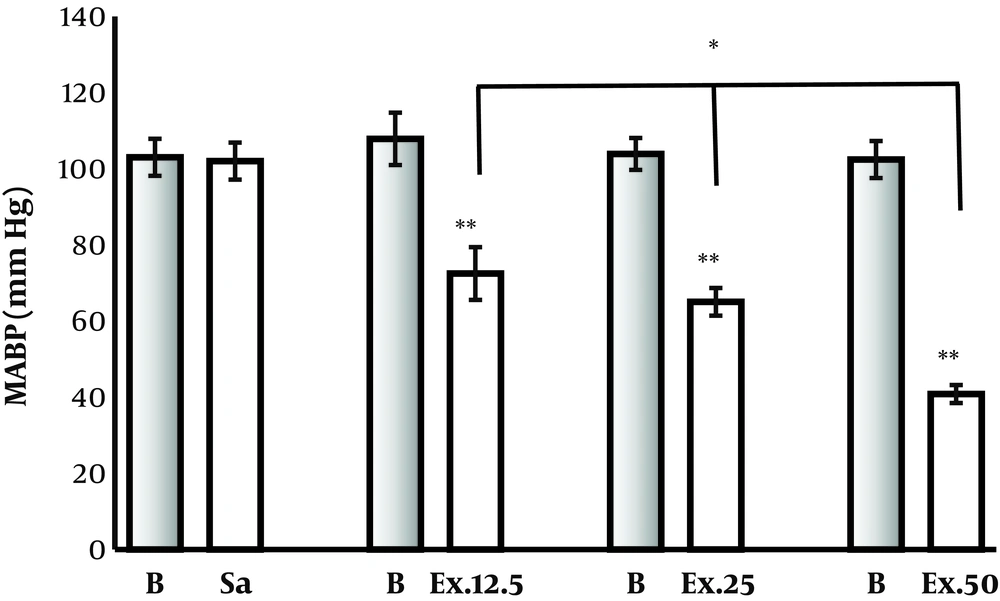
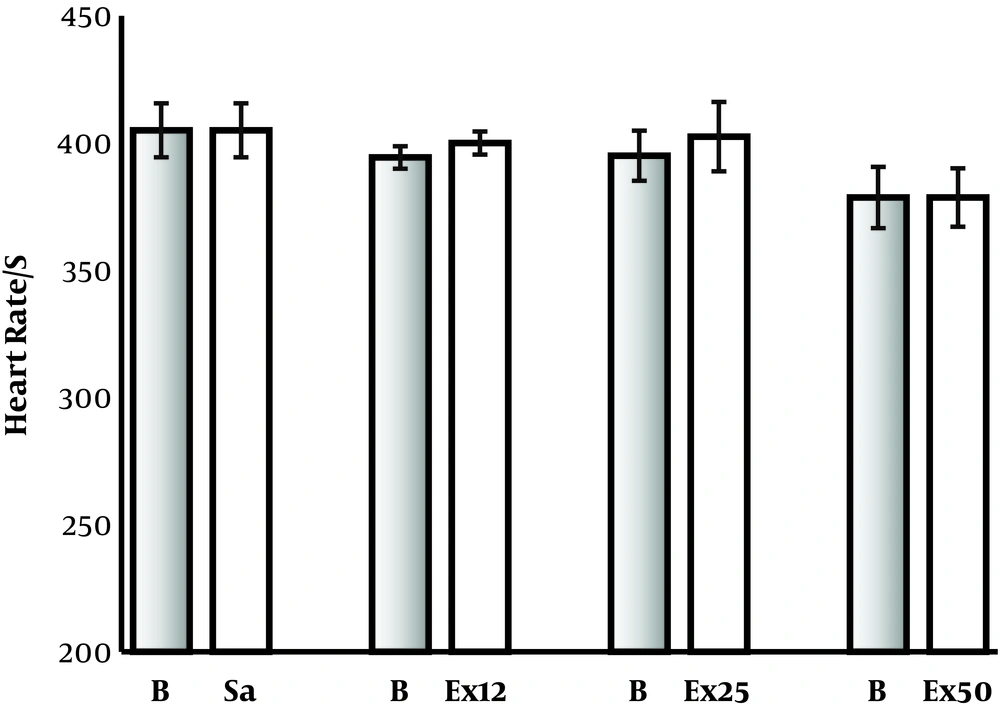
Intravenous injection of P. ferulacea hydroalcoholic extract (12.5, 25, and 50 mg/kg) reduced mean arterial blood pressure (MABP) transiently from 107.8, 103.9, and 102.4 to 72.5, 65, and 40.8, respectively (P < 0.01).Saline injection has no effect on BP of rats. There was a significant difference between the effect of 12.5 and 50 mg/kg of extract (P < 0.01), (Figure 1). The P. ferulacea hydroalcoholic extract had no significant effect on the heart rates (Figure 2).
Effect of hydroalcoholic extract of P. ferulacea on mean arterial blood pressure (MABP) of normal rats. The extract was injected intravenously with 3 doses that reduced MABP significantly. Ex12.5, Ex25, and Ex50 are extract of P. ferulacea at doses of 12.5, 25, and 50 mg/kg, respectively. ** P < 0.001, different doses of extract compare to their baseline (B); *P < 0.05, Ex.50 compare to doses of 12.5 and 25 mg/kg.
Effect of intravenous injection of hydroalcoholic extract of P. ferulacea on heart rate (HR) of normal rats. The extract had not significant effect on the HR of normal rats. Ex12.5, Ex25, and Ex50 are hydroalcoholic extract of P. ferulacea at doses of 12.5, 25, and 50 mg/kg respectively. B, baseline.
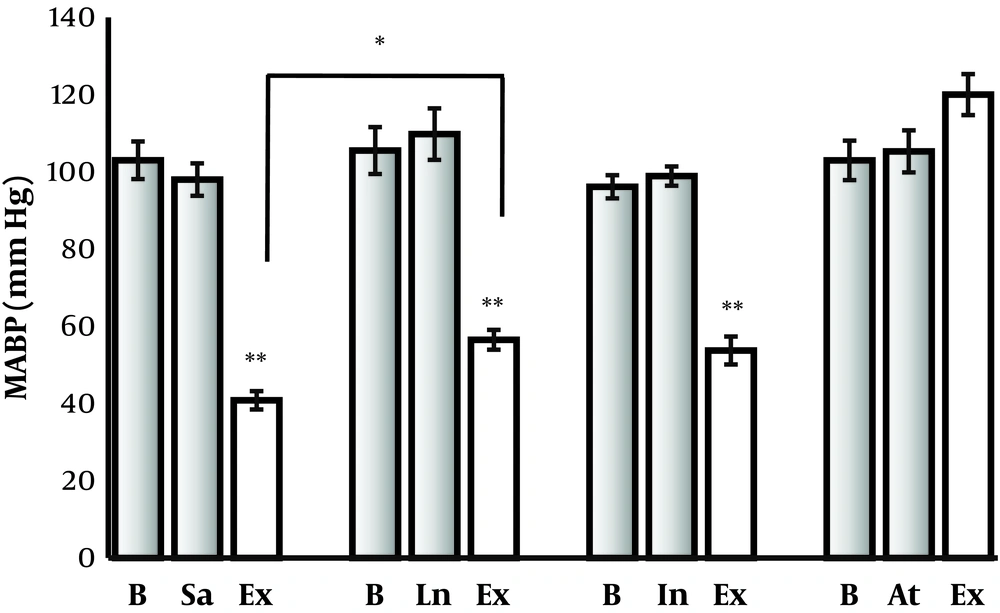
To assess the mechanism of P. ferulacea extract effect on BP, L-NAME (4 mg/kg), or atropine (1 mg/kg), or indomethacin (5 mg/kg) or saline were injected intraperitoneally in different normal rats which had no significant effect on basal BP. About 10 minutes later, P. ferulacea hydroalcoholic extract (50 mg/kg) was injected intravenously. BP had decreased significantly in L-NAME (P < 0.001), indomethacin (P < 0.01) and saline groups (P < 0.001), but hypotensive effect of P. ferulacea extract was eliminated completely by atropine (Figure 3). Comparison between saline and L-NAME groups has shown hypotensive effect of P. ferulacea in L-NAME groups was lower than saline group (P < 0.05).
Evaluating the mechanism of hypotensive effect of P. ferulacea extract on mean arterial blood pressure (MABP) of normal rats. The extract of P. ferulacea reduced MABP significantly (**P < 0.001), but its hypotensive effect was reduced by L-NAME (P < 0.05) and completely eliminated by atropine.
4.2. Chronic Dietary Effects of P. ferulacea on BP of Normotensive and Hypertensive Rats
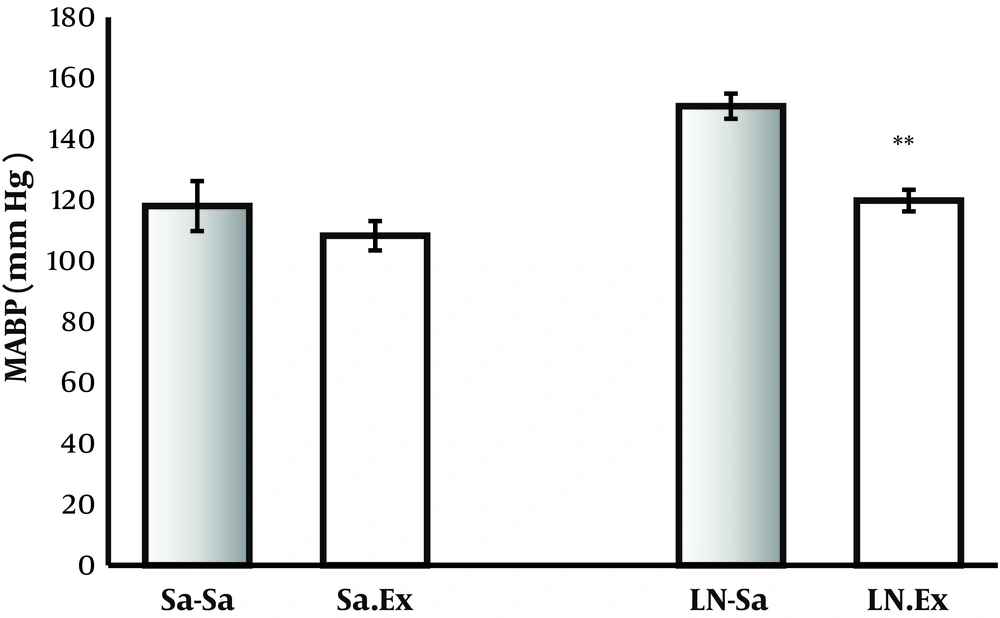
In chronic groups, L-NAME (40 mg/kg/day) prescription increased BP significantly from 112 mm Hg in control group to 149 in hypertensive group (P < 0.01). Oral administration of P. ferulacea extract (500 mg/kg/day) in the hypertensive group reduced BP significantly to 119 mmHg (P < 0.01). However, oral extract (500 mg/kg/day) has no significant effect on BP in the normotensive group (Figure 4).
Chronic nutritional effects of P. ferulacea extract (500 mg/kg) during 4 weeks on mean arterial blood pressure (MABP) of normal and L-NAME- induced hypertensive rats in control and treatment groups (* P < 0.001). The extract did not have significant effect on the MABP of the normal rats, but it prevented the increase in BP induced by L-NAME. Sa, saline; Ex, extract; LN, L-NAME.
4.3. Chronic Dietary Effect of P. ferulacea on the Anxiety Behavior in Rats
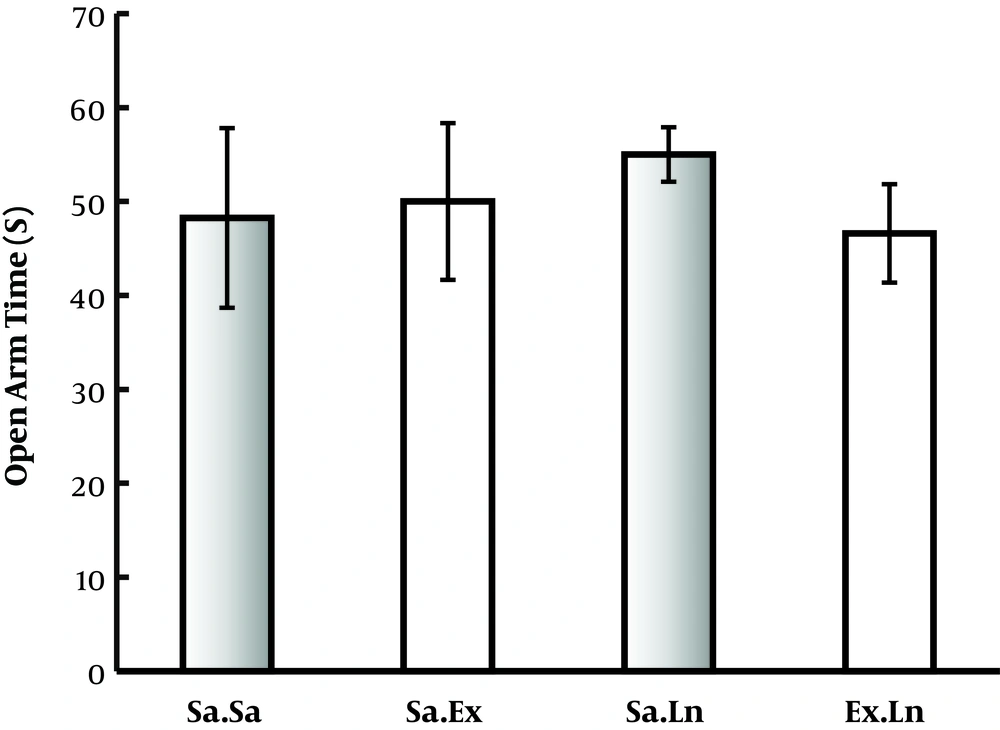
Animals in chronic groups were evaluated for anxiety behaviors by plus maze method in the 27th day of experiment. No significant difference was seen between groups in total time spent in open arm (index of anxiety behaviors) and number of interties (Figure 5).
5. Discussion
Our results show intravenous injection of P. ferulace at doses of 12.5, 25, and 50 mg/kg, reduces MABP of normal rats by 32.7, 37.4, and 60.1%, respectively without significant effect on heart rate. This is the first report about the effect of P. ferulace relaxant in the vessels. In agreement with this study, it has been reported that the acetonic extract of P. ferulacea and the osthole relax the smooth muscles of the uterus and the ileum (6, 7).
Possible mechanisms of P. ferulace effect on BP were examined by a number of factors that have effects on vascular tone. Nitric oxide is an important factor released by endothelial cells and is involved in vascular relaxation and modulation (9). Accordingly, L-NAME was administered intraperitoneally and subsequently the extract of P. ferulace was given intravenously. L-NAME had no significant effect on basal BP, but decreased the hypotension of the extract compared to the saline group. The extract of P. ferulacea appears to relax the blood vessels with NO release.
Endothelial cells produce PGI2 that is inhibited by indomethacin. Administration of indomethacin had no significant effect on hypotension induced by P. ferulace extract.
Cholinergic neurons have also been reported in the cerebrovascular and skeletal vessels, and acetylcholine can cause vasodilatation through endothelium and NO release (12, 13). Intraperitoneal injection of atropine does not affect the baseline BP, but completely eliminated the hypotensive effect of the extract. This response seems to be mediated by muscarinic receptors; it’s remained for more explanation.
The P. ferulacea is used as a food and flavor of yogurt (7), but its dietary effect on BP is unknown. So, its nutritional effects were evaluated on normal BP and in L-NAME-induced hypertension. In agreement with other studies (9), L-NAME administration increased MABP significantly. Chronic feeding of P. ferulacea extract did not have a significant effect on normal BP, but prevented the increase of BP caused by L-NAME in hypertensive rats. Our results are to some extent in agreement with the study of Ogawa. They have shown that oral administration of osthole extracted from Cnidium monnieri decreases BP in hypertensive rats (8). Osthole is a main coumarin of P. ferulacea, which is reported exist only in the root of the plant (4). Nevertheless, another study has been reported that they have extracted osthole from aerial parts of the P. ferulacea (6). This controversy raises the question of whether osthole existed in our extract. We did not carry out phytochemical analysis on the extract, but the hypotension found in our study indicates that there are compounds in our extract that affect the blood vessels.
D-limonene is a monocyclic monoterpene found in the oils of orange, grapefruit and lemon. It has been reported d-limonene diet for 4 weeks reduces BP in the rats that have previously been treated with L-NAME and high-fat diets (14). Limonene is also found in the aerial parts of P. ferulacea (4), which might reduce BP in this study.
Reactive oxygen species (ROS) are involved in vascular diseases and antioxidant deficiency plays an important role in the development of hypertension (9). Additionally, endothelial dysfunction and vascular remodeling were improved in experimental hypertension by antioxidants (15). The P. ferulacea has potent antioxidants activity because of coumarines, alkaloids, flavonoids, terpenoids, and phenolic compounds (4, 5, 16).Consequently, P. ferulacea possibly affected the BP of hypertensive rats due to its antioxidant activity (4, 5). Also flavonoids inhibit angiotensin converting enzyme (17), a main target of anti-hypertensive drugs.
We used the extract concurrently with L-NAME prescription, if the treatment began after the stabilization of hypertension, we could have more accurately checked the effect of the extract on BP. This may be mentioned as one of the study’s faults.
The P. ferulaceais used for different purposes, such as sedative effects (3, 4). Some studies indicate that hypertension is associated with anxiety (15, 18). Is the effect of P. ferulacea hypotension due to its sedative effects? So the effect of extract on anxiety behavior was assessed in chronic groups of rats by elevated plus maze. Our results did not show a significant difference between the times spent in the open arm among the different groups. Also no significant difference was seen in number of entry to the open arms between groups. The absence of rat in the open arm is an index of anxiety behavior. Therefore, the effect of P. ferulacea on BP due to its anxiolytic effects cannot be deduced.
5.1. Conclusions
The present study provides evidence that the hydroalcoholic extract of P. ferulacea has hypotensive effect in normal rats that may be mediated by the muscarinic receptor. In addition, chronic dietary P. ferulacea has prevented the increase in BP induced by L-NAME in rats. So the P. ferulacea as a food supplement seems to be useful for prophylaxis of hypertension.