1. Background
Long-chain unsaturated fatty acids are beneficial for human health. Poultry meat has high contents of polyunsaturated fatty acids. However, increasing the amount of unsaturated fatty acids increases the sensitivity of chicken fat to oxidative degradation, which changes the taste and nutritional composition of the meat. Consumer demand to reduce synthetic preservatives increased the interest in the antioxidant and antimicrobial properties of medicinal herb extracts (1, 2). Free radicals are one of the most important factors in the oxidation of food, which cause oxidation of fats and oils. In addition, byproducts of oxidation of lipids can have negative effects on the food components. As a result, degradation of vitamins and essential fatty acids and production of toxic compounds in food products can lead to undesirable consequences such as inflammatory diseases, cancer, and immunodeficiency in the human body (3). Therefore, the use of antioxidants in order to slowdown the oxidation process in foodstuff and extend the lifetime of opened food products seems necessary. The replacement of phytogenic feed additives (PFAs) with chemical preservatives raised much attention it reduces the adverse effects caused by the consumption of chemical preservatives. Medicinal plants are economic supplements that can be used in poultry farming and their antioxidant effects are evident in the phenolic compounds (3, 4). Heracleum persicum and Pimpinella anisum L. are two medicinal plants from the Apiaceae family with numerous applications in food and drug industries (5). H. persicum, commonly known as Persian hogweed, is recognized as a native flowering plant in Iran (6). There are generally 10 hogweed species, four of which are endemic to Iran (7). The main constituents of hogweed seed include trans-anetole, hexyl butyrate, octyl acetate, and hexyl isobutyrate (8). Research on hogweed suggests its beneficial effects on growth (9-11), immune function (12, 13), hematological parameters (14), and intestine morphometrics (9). Studies also show that addition of hogweed to diet can prevent oxidative changes by free radicals and other reactive species through radical scavenging and lipid peroxidation inhibition (15). On the other hand, anise is described as a potential natural growth promotor for poultry (16), which can be used as an antibiotic alternative. Anise seeds contain different constituents including trans-anetole, eugenol, anisaldehyde, polyacetylenes, methyl chavicol, scopoletin, estragole, coumarins, umbelliferone, terpene hydrocarbons, estrols, and polyenes (8). In addition, some of the positive effects of anise seeds on animals’ performance are attributed to their favorable effects on growth (17-19), antioxidant status (20), ileal nutrient digestibility (21), anti-inflammatory responses, and immune system (22). Also, Hong et al., (23) showed that in chickens fed with anise, the breast muscles had more tenderness in comparison with the antibiotic and control groups.
2. Objectives
The present study aimed at evaluating the effects of the inclusion of hydroalcoholic extract of hogweed or anise in diet on broiler meat quality, immune responses, and intestinal microflora and morphology.
3. Methods
3.1. Preparation of Extracts and Diets
The hogweed and anise seed extracts were purchased from a reputable corporation (Know Tech. Pharmaceutical Co. Tehran, Iran). The entire content of each extract was measured using the Folin-Ciocalteu reagent (24). The hogweed and anise extracts contained 42.27% and 35.80% phenolic compounds, respectively. In total, 400 male Ross 308 chicks (42 ± 3.0 g; one day old) were supplied by Toyoorbarekat Company (Tehran, Iran). The chicks were assigned to five groups based on a completely randomized design in four replicates (20 chicks per replicate). During the experiments, the chickens had free access to water and food in mash form. To meet their nutritional needs, three isocaloric and isonitrogenous dietary formulations were used for the starter (1 - 10), grower (11 - 24), and finisher (25 - 42) periods, as recommended by National Research Council (Table 1). No feed additives were used in the control group, whereas the 2nd, 3rd, 4th, and 5th groups received 100 mg/kg of probiotics (PrimaLac® 454; Star-Labs Clarksdale, USA), 200 mg/kg of hogweed extract, 200 mg/kg of anise extract, and 200 mg/kg of oxytetracycline 20% (Fidax®; Darusazan Iran Co., Tehran, Iran), respectively. In the present study, PrimaLac® 454 feed grade, a multi-strain powder preparation (1 × 108 CFU/g), contained Lactobacillus casei, Bifidobacterium thermophilum, Enterococcus faecium, and Lactobacillus acidophilus (all at 2.5 × 107 CFU/g). This preparation was selected considering the variability and availability of bacterial strains such as Lactobacillus species (25).
| Period | |||
|---|---|---|---|
| Starter (1 - 10 d) | Grower (11 - 24 d) | Finisher (25 - 42 d) | |
| Ingredient (%) | |||
| Corn (8.5% CP) | 54.32 | 58.00 | 66.00 |
| Soybean meal (44% CP) | 39.80 | 35.22 | 29.00 |
| Soya oil | 2.15 | 3.30 | 2.00 |
| Oyster shell | 0.8 | 0.70 | 0.65 |
| Di-calcium phosphate | 1.75 | 1.65 | 1.30 |
| Mineral premixa | 0.25 | 0.25 | 0.25 |
| Vitamin premixa | 0.25 | 0.25 | 0.25 |
| Salt | 0.23 | 0.23 | 0.20 |
| DL-methionine | 0.28 | 0.20 | 0.20 |
| Lysine | 0.17 | 0.20 | 0.15 |
| Anise extractb (the 2nd diet) | 0.02 | 0.02 | 0.02 |
| Hogweed extractc (the 3rd diet) | 0.02 | 0.02 | 0.02 |
| Oxytetracycline antibioticd (the 4th diet) | 0.02 | 0.02 | 0.02 |
| Probiotic primalac®e (the 5th diet) | 0.01 | 0.01 | 0.01 |
| Chemical analysis | |||
| Metabolizable energy (kcal/kg) | 2980 | 3100 | 3150 |
| Crude protein (%) | 23.00 | 21.24 | 19.08 |
| Methionine (%) | 0.51 | 0.50 | 0.47 |
| Methionine + cysteine (%) | 0.95 | 0.81 | 0.76 |
| Lysine (%) | 1.29 | 1.23 | 1.04 |
| Threonine (%) | 0.80 | 0.71 | 0.63 |
| Tryptophan (%) | 0.35 | 0.26 | 0.22 |
| Calcium (%) | 0.98 | 0.90 | 0.76 |
| Available phosphorus (%) | 0.46 | 0.44 | 0.37 |
| Sodium (%) | 0.20 | 0.20 | 0.20 |
Composition and Analysis of Experimental Diets in Different Periods
3.2. Meat Quality
On day 42 of the trial, three broiler chicks were selected per replicate according to their average body weight after 12 hours of fasting. The animals were sacrificed through cervical dislocation, followed by bleeding. After removing the breast muscles, they were stored separately in plastic bags to examine meat quality (e.g., malondialdehid (MDA) content, pH, and water holding capacity (WHC) at 0, 24, and 48 hours after slaughtering at 4°C. The breast muscle pH was measured at 2.5 cm below the surface with a pH meter model pH-211 (Hanna Instruments Inc., Italy) equipped with a spear electrode. Moreover, MDA content, recognized as an indicator of lipid oxidation, was measured in breast muscles using thiobarbituric acid method described by Witte et al. (26). Also, after placing 0.5 g breast meat samples in aluminum foils between metal plates, WHC was measured by leaving the sample under water for one minute at a pressure of 130 bar (27).
3.3. Immune Responses
In each replicate, two chicks were randomly selected on day 28 of age and received peritoneal injections of 7% sheep red blood cell (SRBC) suspension (0.5 mL). Seven days following the SRBC challenge, blood samples were collected. According to the microhemagglutination technique suggested by Qureshi and Havanstein (28), the level of anti-SRBC antibody was measured. The antibody data are presented as log2 of the reciprocal of the highest dilution yielding visible agglutination.
3.4. Intestinal Morphometry
At the end of experiment (42 days), two chicks were randomly sacrificed per replicate, and the intestinal middle segments (jejunum) were separated. Almost 1.5 cm of the intestine was sampled and positioned for 24 hours after washing with 85% saline; then, the samples were placed in formalin 10%. After preparation of the samples, a light microscope (Olympus Co. Tokyo, Japan) equipped with a computer was used to measure the villus width, villus height (from the villus tip to the villus-crypt junction), villus height to crypt depth ratio (VH/CD), and crypt depth (invagination depth between the adjacent villi), according to a method described by Sakamoto et al. (29). The villus height and width were determined to calculate the villus surface area:
Where, W represents the villus width and L denotes the villus height.
3.5. Ileal Bacterial Populations
On day 42 of the trial, to investigate the ileal bacterial populations, 1 g of ileal content of two randomly selected chicks per replicate was added to a sterile physiological salt solution (9 mL), and bacterial populations were examined based on the method described by Mookiah et al. (30). Bacterial populations are expressed as log10 CFU/g of ileal content. It should be noted that all bacterial enumerations were carried out in duplicate.
3.6. Statistical Analysis
For statistical analysis, the general linear model was applied in SAS (31). A randomized design was applied in ANOVA. Duncan’s multiple-range test was performed to measure the mean values (32). P < 0.05 was considered the level of significant.
4. Results
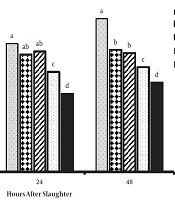
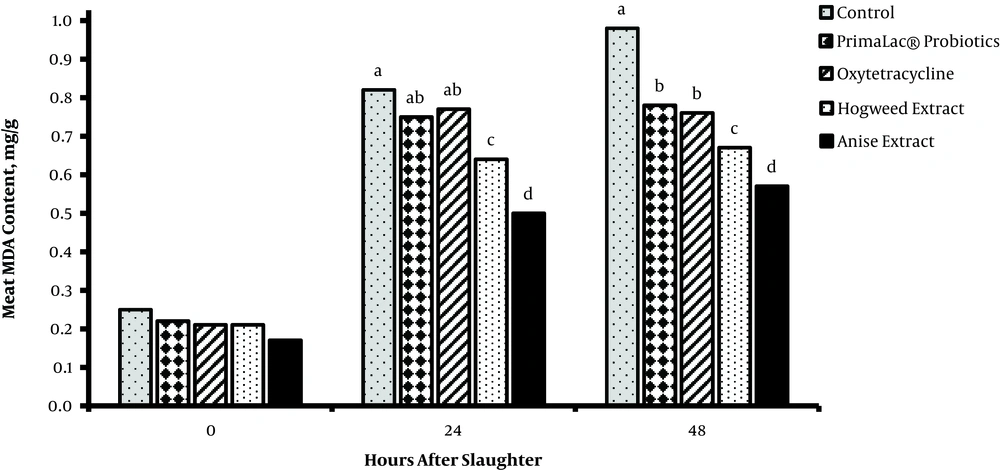
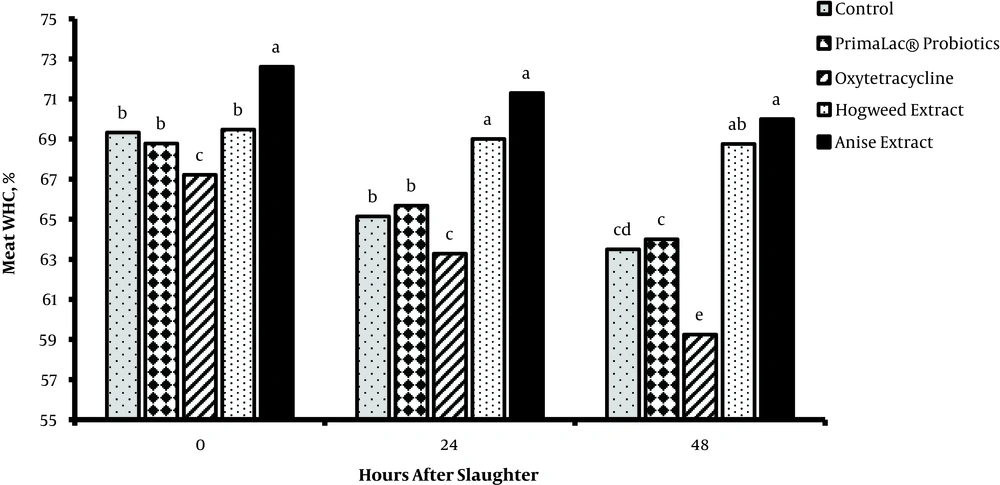
The results related to meat quality (pH, MDA content, and WHC) of breast muscles are presented in figures 1, 2, and 3. After meat collection, the results indicated that pH was not majorly influenced by treatments (P > 0.05), whereas the MDA content (24 and 48 hours) was significantly lower in groups fed with PFAs, compared to the other groups (P < 0.05). Also, supplementation of diet with 200 mg/kg hogweed and anise extracts increased the meat WHC (P < 0.05).
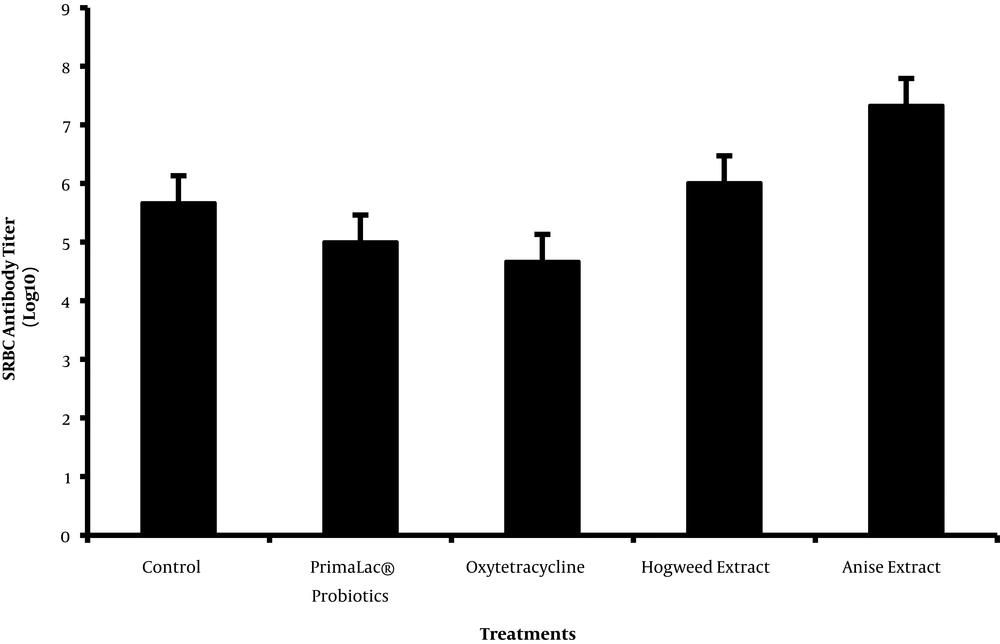
Figure 4 presents the effects of the supplementation of diet with feed additives on humoral immunity (anti-SRBC titer). Based on the findings, treatments did not affect the immune responses.
The effects of experimental treatments on the ileum microbial population and intestinal morphology are shown in Table 2. In comparison with the controls, a significantly lower ileal content of coliforms was observed in the treatment groups (P < 0.05). Also, ileal Escherichia coli and total aerobic bacteria counts were significantly lower in the hogweed extract group in comparison with the other groups (P < 0.05). Moreover, the results showed that treatments did not influence the crypt depth or villus width (P > 0.05). The treatment groups showed significantly higher villus height, VH/CD, and villus area in comparison with the controls (P < 0.05).
| Trait | Treatment | Statistical Parameter | |||||
|---|---|---|---|---|---|---|---|
| Control | PrimaLac® Probiotics | Oxytetracycline | Hogweed Extract | Anise Extract | SEM | P Value | |
| Intestinal microflora log10 (CFU/g) | |||||||
| Total of aerobic bacteria | 10.49a | 7.95b | 9.43a | 6.21c | 8.47a, b | 0.37 | 0.04 |
| Escherichia coli | 3.53a | 3.43a | 3.73a | 1.87b | 3.63a | 0.14 | 0.03 |
| Coliform bacteria | 6.52a | 4.68b | 2.22c | 2.16c | 4.79b | 0.54 | 0.01 |
| Morphology | |||||||
| Villus height (µm) | 1046.00c | 1217.4a, b | 1134.6b | 1193.6a, b | 1224.2a | 26.85 | 0.001 |
| Villus width (µm) | 120.20 | 114.8 | 112.60 | 120.40 | 107.20 | 5.09 | 0.347 |
| Crypt depth (µm) | 119.00 | 112.20 | 112.60 | 110.00 | 122.40 | 5.60 | 0.532 |
| VH/CDd | 8.79b | 10.85a | 10.08a | 10.85a | 10.00a | 0.376 | 0.011 |
| Villus area (mm2) | 0.455b | 0.495a, b | 0.460a, b | 0.500a | 0.477a, b | 0.015 | 0.024 |
Effects of the Inclusion of PrimaLac® Probiotics, Oxytetracycline, and Hogweed and Anise Extracts in Diet on Intestinal Microflora and Morphology of Broilers
5. Discussion
In recent years, improvement of meat quality using plant extract supplementations is highlighted in poultry farming since consumers are concerned about poultry meat quality. Reduction of meat pH causes reduced WHC, and as a result the nutritional value of meat, which depends on the amount of protein denaturation in tissue, is eliminated. The current study did not find significant effects of treatment on meat pH, but WHC was higher than those of the medicinal herb extract groups in agreement with the result of Jiang et al. (33). The meat oxidative stability may be affected by feeding and production conditions of animals (33). The total phenol content of the breast meat of birds receiving diets containing supplements was significantly different and has higher antioxidant activities compared to those of controls (3). Supplementation of diet with 200 mg/kg hogweed or anise extract slightly decreases the MDA content of meat (P < 0.05). MDA, as the end product of lipid peroxidation, is an index for ROS (reactive oxygen species)-induced biological damage (34), isolated from urine, blood, and tissue. Therefore, PFA supplements are speculated to reduce lipid peroxidation and consequently increase meat quality. However, the effects of supplements with hogweed and anise extracts on meat lipid peroxidation and quality are not examined in broiler chickens. Accordingly, PFA supplements are potential additives to improve meat quality in broiler chickens. Amad et al. (35), reported that broilers fed phytogenics containing essential oil of star anise as lead active components had an improved feed-conversion ratio and such effects can result in higher economic efficiency in broiler meat production. The consequences of poultry production for the environment, food safety, and animal welfare issues are now part of consumers’ opinions and demands. Replacement strategies based on novel or alternative feeding practices without antibiotics are favored by the industry.
SRBC antibody titer was unaffected by either probiotic, antibiotic, or PFAs supplementation (Figure 4). Previous studies show that supplementation of diet with PFAs stimulates cellular and humoral immune functions (10, 11, 36), which might be attributed to its content in furanocoumarins and flavonoids. Amresh et al. (37), showed that flavonoids and polyphenolic compounds help the immune system through antioxidant activity. The improved immune function by prebiotics supplementation can be attributed to preferential colonization of beneficial bacteria and microbial products that produce immune response. On the contrary, Eftekhari et al. (9) observed that broiler diet supplemented with hogweed did not have any significant effects on primary and secondary antibody responses. Furthermore, Hong et al. (23), reported that total antibody titer was not affected by the commercial blend of phytogenics supplementation, which was similar to the current study findings regarding PFAs supplementation.
Based on the results of intestinal microflora and morphology analysis, administration of synthetic (PrimaLac® probiotics and oxytetracycline) or natural (hogweed and anise extracts) food additives improves the poultry health and productivity by modulation of intestinal microbial ecosystem. Inclusion of prebiotics (38) and PFAs (39) in diet enriched beneficial microbiota and decreased pathogenic bacteria colonization (e.g., E. coli). In a nutshell, medicinal plant extract supplements are economic alternatives, which can also be added to poultry diet in order to enrich intestinal microbiota.
In diets containing PFA, villus height, VH/CD, and villus area were notably higher in broiler chicks fed with synthetic (PrimaLac® probiotics and oxytetracycline) or natural (hogweed and anise) food additives, which promote the absorption of nutrients. The present study results were not in congruence with earlier reports, which indicated that supplementation of diet with plant extract increased the villus height (23) and VH/CD ratio (9, 11). Moreover, Amad et al. (21), showed that PFA supplementation causes jejunal morphological changes, which may affect the absorption of nutrients and improve feed conversion ratio (FCR). In another study by Viveros et al. (40), phenolic compounds could change the intestinal microflora and morphology and improve the intestinal bacteria biodiversity in broiler chicks. Also, a recent report by Mohiti-Asli and Ghanaatparast-Rashti (39) showed that oregano essential oil and phytogenic blend had similar antimicrobial activities, despite differences in their effects, on the intestinal morphological features. Therefore, adding phytogenics to broiler diet should be relied on the histomorphometry results of the small intestine rather than antimicrobial activities. In sum, it can be stated that PFA supplementation synergistically improves weight gain and FCR by modulating the intestinal system.
5.1. Conclusions
The present study results indicated that hogweed and anise extracts could be potential natural promoters of poultry growth and proper probiotic and antibiotic alternatives. Addition of PFAs had more significant effects on meat quality, compared to antibiotics and probiotics. Therefore, it is suggested to include 200 mg/kg PFAs in the diet of broiler chicks to improve the intestinal morphology and decrease harmful bacterial populations in the ileum. However, the results of different studies on supplementation of broiler diet with hydroalcoholic extract of hogweed and anise are still controversial, which could be due to the differences among various medicinal plants and their compositions as well as housing condition and health stage of examined animals. According to these variables, diets supplemented with 200 mg/kg extract of hogweed or anise were suitable to improve the broiler meat quality. Due to the fact that the report on supplementation with hogweed or anise and their active components in broilers is limited, the hogweed or anise and their active components could serve as a novel nutritional means of improving broiler meat quality, immune responses, and intestinal microflora and morphology. These effects can result in higher economic efficiency in broiler meat production. More detailed studies are still needed to elucidate the mode of action of hogweed and anise ingredients on nutritional and physiological response of broilers under various circumstances.