1. Background
Muscari Miller, from the Asparagaceae family, is represented by about 50 species worldwide. These ornamental plants are distributed in Iran, Iraq, Anatolia, Syria, Caucasia, Central and East Southern of Europe, Southern Russia, and Africa (1-3). Eight bulbous species were reported in Iranian flora (2, 4, 5).
Previous literature offered various medicinal and biological effects such as a diuretic, emetic, anti-oxidant, anti-inflammatory, hypoglycemic, and stimulant effects (2, 6, 7).
Moreover, earlier phytochemical studies on the plants of genus Muscari showed the occurrence of flavonoid, alkaloid, terpenoid, and steroid structures (4). Muscari genus is one of the chief sources of homoisoflavonoid structures, which are a subclass of flavonoids and are rarely found in nature (5, 8-10). In a genotoxicological study, Miadokova et al. (8), suggested that homoisoflavonoids from the bulbs of Muscari racemosum (L.) have important pharmacological effects and might be beneficial for inhibition of cancer owing to their anti-mutagenic and anti-clastogenic properties.
Approximately, 240 types of natural occurring homoisoflavonoids were reported from the various parts of the plants from Asparagaceae, Fabaceae, Polygonaceae, Orchidaceae, Portulacaceae, and Gentianaceae families. They have an extensive variety of biological effects including anti-oxidant, anti-microbial, anti-viral, anti-mutagenic, anti-diabetic, anti-inflammatory, anti-angiogenic, vasorelaxant, immunomodulatory, and cytotoxic effects (5, 11). In addition, according to previous knowledge, homoisoflavonoid structures can be a good source of ingredients with anti-plasmodial effects (12).
2. Objectives
The present study aimed to evaluate some biological activities and phytoconstituents of Muscari inconstrictum Rech. f. bulbs. To the best of our knowledge, this is the first study on phytochemicals and biological effects of M. inconstrictum.
3. Methods
3.1. Plant Material
Muscari inconstrictum Rech. f. was collected from the gardens of East-Azerbaijan, the province of Iran in April 2016. After authentication, the voucher specimen (no.: 8897) was stored at the Herbarium of the East-Azarbaijan Agricultural and Natural Resources Research and Education Center, Tabriz, Iran.
3.2. Extraction
Air-dried bulbs of M. inconstrictum were crushed and were Soxhlet-extracted with n-hexane, chloroform, and methanol (MeOH), continuously. Subsequent extracts were evaporated at 50°C using a rotary evaporator.
Moreover, the 100 g of powdered bulbs of M. inconstrictum was subjected to hydro distillation for four hours using a Clevenger device. The produced essential oil was measured (V/W) and dried via anhydrous sodium sulfate, and then stored in a sealed vial for further analysis.
3.3. Fractionation
About 0.7 g of chloroform extract and 0.5 g of n-hexane extract from M. inconstrictum bulbs were fractionated by vacuum liquid chromatography (VLC) over silica gel (20 g) with solvent mixtures of increasing ratios of polarities to ethyl acetate/n-hexane (i.e., 10:90, 20:80, 40:60, 60:40, 80:20, and 100:0) and MeOH. All the fractions were completely dried using a rotary evaporator at a maximum temperature of 45°C (13).
3.4. Free Radical Scavenging Activity
The concentrated n-hexane, chloroform, MeOH extracts, and VLC fractions of the most potent extract were objected to anti-oxidant assay test using DPPH (Sigma, Germany) as a reagent and quercetin as a positive control (14, 15). DPPH (4 mg) was dissolved in 50 mL methanol for methanolic extract and chloroform (for n-hexane and chloroform extracts) to obtain the stock solution. Methanolic extract was dissolved in methanol (1 mg/mL). Moreover, n-hexane and chloroform extracts were dissolved in chloroform (1 mg/mL). Concentrations of 2.5 × 10-1, 1.25 × 10-1, 6.25 × 10-2, 3.13 × 10-2, and 1.56 × 10-2 mg/mL were made by dilution. About 2 mL of each dilution was mixed with DPPH (2 mL). Tubes were kept at room temperature for 30 min to allow any reaction to occur. The absorbance of samples was measured using UV/Visible Spectrophotometer (Spectronic Genesys spectrophotometer, USA) at 517 nm. The same procedure was repeated three times and the average absorbance was recorded. The reduction percentage of DPPH (R%) was calculated as below and the result was reported as sample concentration providing 50% DPPH reduction (RC50).
Abs, absorbance of samples.
3.5. Cell free β-Hematin Formation Assay
The anti-malarial activity of the three extracts was evaluated by the heme biocrystallization technique described by Afshar et al. (16). Various concentrations of extracts (0.4 - 2 mg/mL in dimethyl sulfoxide) were mixed with 100 µL of hematin (dissolved in 0.1 M NaOH), 10 mM oleic acid, and 10 µM HCl. The reaction volume was attuned to 1000 µL by sodium acetate buffer (pH = 5). The microtubes were incubated at 37°C for 24 h with constant shaking. Afterward, samples were centrifuged (12,000 rpm for 10 min) and the hemozoin sediments were washed repetitively in 2.5% (w/v) sodium dodecyl sulfate (SDS) in phosphate buffered saline. Finally, they were washed in sodium bicarbonate (0.1 M and pH = 9.0) until the supernatant was clear (after 4-6 washes). In the next step, the supernatant was eliminated and the sediments of hemozoin were re-suspended using 1 mL of NaOH (0.1 M). The absorbance of the samples was measured at 400 nm with UV/Visible Spectrophotometer (Spectronic Genesys spectrophotometer, USA). The results were informed as inhibition percentage (I %) of heme crystallization calculated as follows:
3.6. Identification of Components
3.6.1. GC-MS (Gas Chromatography-Mass Spectrometry) Analysis
GC-MS and gas chromatography with flame ionization detector (GC-FID) analysis were performed on a Shimadzu QP-5050A GC-MS system (Japan) and GC-17A equipped with a DB-1 fused silica column (60 m × 0.25 mm i.d.; 0.25-µm film thickness). Helium was used as the carrier gas at a flow rate of 1.3 mL/min. The sample was diluted 1:10 in n-hexane and 1 µL was injected into the column. Split ratio, scan time, ionization energy, and acquisition mass range were 1:33, 1 s, 70 eV, and 30 - 600 amu, respectively.
The oven temperature program for essential oil was a temperature of 50°C rising to 260°C at a rate of 3°C /min for a total run time of 75 min. The temperature of injector was set at 220°C and the detector temperature was 260°C. In addition, the temperature program for VLC fractions was an oven temperature of 50°C rising to 300°C at a rate of 4°C /min for a total run time of 74 min. The injector temperature was set at 270°C and the detector temperature was 300°C.
Identification of the volatile constituents were completed based on the comparison of the retention times (Rt) and the data of mass spectral with the (C8-C20) and (C21-C40) standard alkanes from Sigma-Aldrich (USA), computer matching with the WILEY229, NIST21, and NIST 107 libraries, and comparing the fragmentation patterns of the mass spectra with those reported in the texts (17, 18).
3.6.2. Total Phenolic Content (TPC)
Determination of total phenolic content of extracts was executed using folin-ciocalteu (Merck, Germany) as a reagent and gallic acid as a positive control (19). About 1 mL of herbal sample (5 mg/100 mL acetone in water 60:40) was mixed with 0.2 mL Folin-Ciocalteu and 0.5 mL Na2CO3 (2%) and centrifuged at 12000 rpm for five min. After a 30-min incubation at room temperature, the absorbance of each sample was measured at 750 nm with UV/visible spectrophotometer (Spectronic Genesys spectrophotometer, USA). The same procedure was repeated in triplicate and average absorption was documented. TPC was reported as gallic acid equivalent in mg/g of sample.
3.6.3. Total Flavonoid Content (TFC)
The extracts were assessed to determine their total flavonoid content with aluminum chloride reagent and rutin as a positive control (20). About 2 mL of each sample (1 mg/1 mL methanol in water 80:20) was mixed with 1 mL reagent (AlCl3 crystals and sodium acetate crystals in 100 mL of 80% of methanol in water) and 400 µL distilled water. Tubes were permitted to stay at room temperature for 30 min. Subsequently, the absorbance of samples was measured at 430 nm with UV/Visible Spectrophotometer (Spectronic Genesys spectrophotometer, USA). TFC was reported as rutin equivalents/g of sample.
3.6.4. Preliminary Phytochemical Analysis of Fractions
3.6.4.1. Thin Layer Chromatography
The identification of chief chemical groups was carried out by thin layer chromatography (TLC) on silica gel 60 F254 Merck (layer thickness 0.25 mm) as follows:
Toluene:ether (l:l, saturated with 10% acetic acid) and chloroform:ethyl acetate (60:4) were used as a solvent system of 80% and 100% fractions of chloroform extract. Natural products-polyethylene glycol reagent (NP/PEG) and also 1% potassium hydroxide reagent was used as the reagent under UV 366 nm.
Chloroform:glacial acetic acid:methanol:water (64:32:12:8) were used as a solvent system of 40% fraction of n-hexane extract. Anisaldehyde-sulphuric acid was used as the reagent and visualization was after about 10 min at 100°C; they were detected under UV 366 nm (21, 22). Liebermann-Burchard, opened loop-closed loop response, and Shinoda tests were performed for confirmation of TLC analysis.
3.6.4.2. Liebermann-Burchard Test
The fractions are treated with few amounts of acetic anhydride. The mixture was boiled and cooled. Then sulfuric acid was added to the mixture from the sides of the test tube. The creation of a deep red color implies the presence of triterpenoids (23).
3.6.4.3. Opened Loop-Closed Loop Response
About two drops of 1% sodium hydroxide solution were added in the sample tube and were heated in boiling water for three min to get a clear solution. Likewise, about four drops of 2% hydrochloric acid were added to the solution. Here, a cloudy appearance means the presence of coumarins and lactones (24).
3.6.4.4. Shinoda Test
Magnesium ribbon was added to the test solution followed by adding hydrochloric acid. Creation of a pink scarlet, crimson red, or sometimes green to blue color after a few minutes indicates the presence of flavonoids (23).
4. Results
4.1. Free Radical Scavenging Activity
Free radical scavenging activity of the three extracts (n-hexane, chloroform, and MeOH) and VLC fractions of chloroform extract, as the most potent anti-oxidant extract, was evaluated by the DPPH method. Table 1 shows the results of the anti-oxidant assay.
| Sample | Yieldb, % | RC50, mg/mL |
|---|---|---|
| n-hexane extract | 0.45 | 0.48 ± 0.01 |
| Chloroform extract | 0.30 | 0.046 ± 0.005 |
| MeOH extract | 8.21 | 0.53 ± 0.06 |
| 10% VLC fraction of chloroform extract | 2.46 | 0.52 ± 0.07 |
| 20% VLC fraction of chloroform extract | 3.02 | 7.40 ± 1.03 |
| 40% VLC fraction of chloroform extract | 1.70 | 2.13 ± 0.21 |
| 60% VLC fraction of chloroform extract | 2.56 | 0.22 ± 0.009 |
| 80% VLC fraction of chloroform extract | 19.28 | 0.034 ± 0.005 |
| 100% VLC fraction of chloroform extract | 30.06 | 0.038 ± 0.007 |
| MeOH VLC fraction of chloroform extract | 9.26 | 0.028 ± 0.002 |
| Quercetinc | - | 0.0030 ± 0.0004 |
aValues are expressed as mean ± SD.
bThe amount produced from extraction of plant powder using soxhlet apparatus, or fractionation of the extracts using VLC method.
cQuercetin was used as control positive.
4.2. Cell free β-Hematin Formation Assay
As shown in Table 2, the anti-malarial effect of plant extracts was assessed by the heme biocrystallization method. The n-hexane extract as the potent part was exposed to fractionation by the VLC method. The anti-malarial test of seven different polarity fractions showed a significant effect of 40% ethyl acetate/n-hexane fraction with an IC50 value of 0.56 ± 0.01 and IC90 value of 1.30 ± 0.01 mg/mL.
| Sample | Yieldb, % | IC50, mg/mL | IC90, mg/mL |
|---|---|---|---|
| n-hexane extract | 0.45 | 0.68 ± 0.03 | 2.69 ± 0.056 |
| Chloroform extract | 0.30 | 1.12 ± 0.02 | 1.69 ± 0.012 |
| MeOH extract | 8.21 | 2.15 ± 0.02 | 5.71 ± 0.327 |
| 10% VLC fraction of n-hexane extract | 5.33 | 0.81 ± 0.06 | 4.27 ± 0.812 |
| 20% VLC fraction of n-hexane extract | 14.07 | 1.52 ± 0.09 | 11.68 ± 2.207 |
| 40% VLC fraction of n-hexane extract | 13.39 | 0.56 ± 0.01 | 1.30 ± 0.01 |
| 60% VLC fraction of n-hexane extract | 12.02 | -c | -c |
| 80% VLC fraction of n-hexane extract | 9.29 | 1.70 ± 0.254 | 17.52 ± 5.409 |
| 100% VLC fraction of n-hexane extract | 6.69 | 3.24 ± 0.27 | 11.34 ± 1.087 |
| MeOH VLC fraction of n-hexane extract | 4.92 | -c | -c |
| Chloroquinc | 0.04 ± 0.002 | 0.35 ± 0.006 |
aValues are expressed as mean ± SD.
bThe amount produced from extraction of plant powder using soxhlet apparatus, or fractionation of the extracts using VLC method.
c-, No effect.
dChloroquin was used as control positive.
4.3. Phytochemical Screening of Essential Oil
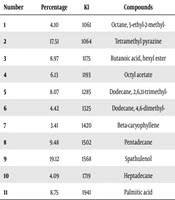
The GC-MS analysis results of the essential oil of M. inconstrictum bulbs are presented in Table 3.
| Number | Percentage | KI | Compounds |
|---|---|---|---|
| 1 | 4.10 | 1061 | Octane, 5-ethyl-2-methyl- |
| 2 | 17.51 | 1064 | Tetramethyl pyrazine |
| 3 | 8.97 | 1175 | Butanoic acid, hexyl ester |
| 4 | 6.13 | 1193 | Octyl acetate |
| 5 | 8.07 | 1285 | Dodecane, 2,6,11-trimethyl- |
| 6 | 4.42 | 1325 | Dodecane, 4,6-dimethyl- |
| 7 | 3.41 | 1420 | Beta-caryophyllene |
| 8 | 9.48 | 1502 | Pentadecane |
| 9 | 19.12 | 1568 | Spathulenol |
| 10 | 4.09 | 1719 | Heptadecane |
| 11 | 8.75 | 1941 | Palmitic acid |
aTotal compounds = 11; total identified = 94.05%; alkanes = 30.16%; fatty acids and derivatives = 23.85%; cyclic sesquiterpenoides = 22.53%; alkylpyrazine structure:17.51%.
Table 4 presents gas chromatographic and mass spectral data of the components of the methanolic fraction of chloroform extract. Moreover, Table 5 shows 40% ethyl acetate/n-hexane VLC fraction of n-hexane extract.
| Number | Percentage | KI | Compounds |
|---|---|---|---|
| 1 | 55.90 | 1270 | Carvacrol |
| 2 | 44.10 | 1944 | Palmitic acid |
aTotal compounds = 2; total identified = 100%; monoterpenoid phenol = 55.90%; fatty acid = 44.10.
| Number | Percentage | KI | Compounds |
|---|---|---|---|
| 1 | 42.84 | 1948 | Palmitic acid |
| 2 | 0.90 | 1980 | Ethyl palmitate |
| 3 | 20.30 | 2186 | Methyl linoleate |
| 4 | 17.91 | 2193 | Oleic acid |
| 5 | 1.04 | 2387 | Tetracosane |
| 6 | 2.24 | 2604 | Hexacosane |
| 7 | 7.91 | 2816 | Octacosane |
aTotal compounds = 12; total identified = 93.14%; fatty acids and derivatives = 81.95%; alkanes =11.19.
Total phenol and flavonoid contents of three main extracts were evaluated by two colorimetric methods correspondingly. Table 6 presents the results of the TPC and TFC assay results.
| Sample | n-Hexane Extract | Chloroform Extract | MeOH Extract |
|---|---|---|---|
| TPC, mg/g | 136.62 ± 3.5 | 809.69 ± 10.3 | 6.28 ± 0.23 |
| TFC, mg/g | 3.15 ± 0.06 | 13.99 ± 0.17 | 6.02 ± 0.12 |
aValues are expressed as mean ± SD.
TLC results indicated the presence of hydroxylated flavan and coumarin (blue to violet-blue zones) structures in the 80% and 100% VLC fractions of chloroform extract and existence of coumarins in a methanolic fraction of chloroform extract as the potent anti-oxidant fractions.
In addition, the appearance of violet spots in TLC analysis suggests the presence of saponin structures in the 40% fraction of n-hexane extract.
Shinoda, opened loop-closed loop response, and Liebermann-Bouchard tests confirmed the presence of flavonoid, coumarin, and saponin structures in the target fractions.
5. Discussion
As the first biological and phytochemical study of M. inconstrictum, we evaluated the anti-oxidant and anti-malarial activities of n-hexane, chloroform, methanolic extracts with different polarities, and the most potent VLC fractions of the bulbs of M. inconstrictum. Furthermore, the chemical components of the liquid essential oil, the potent anti-oxidant fractions, and the strongest fraction in the anti-malarial assay were identified by the GC-MS. Furthermore, other preliminary phytochemical tests were performed for confirmation of the existence of several structural groups.
According to obtained results (Table 1), chloroform extract with RC50 value of 0.046 ± 0.005 mg/mL was the most potent anti-oxidant extract and methanolic (RC50 = 0.028 ± 0.002 mg/mL), 80% ethyl acetate/n-hexane (RC50: 0.034 ± 0.005 mg/mL), and 100% ethyl acetate/n-hexane (RC50 = 0.038 ± 0.007 mg/mL) VLC fractions of chloroform extract were the strongest fractions, in the order of their appearance. In comparison, quercetin, as the positive control, showed an RC50 value of 0.0030 ± 0.0004 mg/mL.
TLC analysis of methanolic, 80% and 100% ethyl acetate/n-hexane fractions of chloroform extract demonstrated the existence of coumarins in methanol fraction and flavonoid and coumarin structures in 80% and 100% ethyl acetate/n-hexane fractions. Shinoda test and opened loop-closed loop response confirmed the existence of flavonoids and coumarins.
The evaluation of total phenolic and flavonoid contents of the extracts also confirmed the positive relation between the phenol and flavonoids contents and the antioxidant effects of the chloroform extract of M. inconstrictum.
In addition, GC-MS analysis of the methanolic fraction of chloroform extract as the most anti-oxidant part showed the presence of carvacrol as a phenolic monoterpenoid and palmitic acid as a saturated fatty acid structure. Carvacrol is a well-known natural anti-oxidant agent, which is known for its antibacterial, antiviral, anti-fungal, antiparasitic (21, 22, 25), anti-carcinogenic (23), analgesic anti-inflammatory properties (26). Palmitic acid also was identified as a selective cytotoxic substance on cancer cell lines and showed in vivo anti-tumor effect in mice (27).
Anti-malarial assay on different extracts and fractions of M. inconstrictum bulbs (Table 2) showed the potent effect of n-hexane extract with IC50 and IC90 values of 0.68 ± 0.03 mg/mL and 2.69 ± 0.056 mg/mL and 40% ethyl acetate/n-hexane VLC fraction of n-hexane extract with IC50 and IC90 values of 0.56 ± 0.01 mg/mL and 1.30 ± 0.01 mg/mL in comparison to chloroquin (IC50 = 0.04 ± 0.002 and IC90 = 0.35 ± 0.006 mg/mL).
The presence of saponins was established by TLC analysis and Liebermann-Bouchard tests in 40% ethyl acetate/n-hexane VLC fraction of n-hexane extract. Previous studies reported that the saponin structures could have an antiplasmodial activity (28, 29).
Moreover, GC-MS analysis of the mentioned fraction for the identification of volatile part of fraction demonstrated the existence of fatty acid derivatives as the main groups of components. According to the previous investigations, it seems that removing the fatty acid and lipid structures can cause stronger anti-malarial effects (28).
Malaria parasite uses red blood cells as a nutrition source and heme is a byproduct of the hemoglobin degradation process. Produced heme interrupts the vital functions of parasite and parasite forms hemozoin crystals in acidic vacuoles as a detoxification technique. Therefore, inhibition of transforming heme to hemozoin has been considered as the main target of anti-malarial medications (13, 30). It seems that the presence of saponin and phenolic terpenoid compounds in the chloroform extract inhibits the conversion of heme to hemozoin and is responsible for anti-malarial effects.
In the next step, the phytochemical evaluation of essential oil of M. inconstrictum bulbs using GC-MS instrument represented the existence of 11 components with about 94.05% of the total components of oil. Koats index and relative area percentage of the identified compounds are listed in Table 3. Spathulenol and tetramethylpyrazine were the two main ingredients of volatile oil. Spathulenol, as a tri-cyclic sesquiterpene alcohol, could be responsible for some biological activities such as anti-inflammatory, anti-oxidant, anti-proliferative, anti-microbial and as a mosquito repellant agent against Aedes aegypti and Anopheles stephensi in the essential oil of various plant species (31). Moreover, tetramethylpyrazine is the second major structure of M. inconstrictum bulbs essential oil that was reported as a remedy of neurovascular disorders and ischemic stroke. It can inhibit the aggregation of platelet, reduce the viscosity of blood, progress the microcirculation, and increase cerebral and coronary blood flow. It was also described as an anti-bacterial and protective agent against focal cerebral ischemia/reperfusion injury in rats (32, 33).
The other major components of the oil are pentadecane (9.48%), butanoic acid, hexyl ester (8.97%), palmitic acid (8.75%), and dodecane, 2,6,11-trimethyl (8.07%) with alkane and fatty acid structure.
There are considerable differences between the phytochemicals of M. neglectum aerial parts oil as the other species of Muscari genus, which was characterized by bis (2-ethylhexyl) phthalate (18.6%), tributyl(methyl)stannane (10.1%), and bis(chlorophenyl) sulphone (8.7%) (4).
However, according to our findings and previous knowledge, it is necessary to focus on the study of M. inconstrictum pure compounds and their biological activities.
5.1. Conclusions
Based on the results of the present preliminary phytochemical and biological investigation, among different fractions of M. inconstrictum bulbs, methanolic, 80% and 100% ethyl acetate/n-hexane VLC fractions of chloroform extract showed significant anti-oxidant activity, which can be related to the presence of flavonoid and coumarin structures. Moreover, 40% of ethyl acetate/n-hexane VLC fraction of n-hexane extract with saponin structures is introduced as the most potent anti-malarial part.
GC-MS analysis of methanol fraction of chloroform extract, 40% ethyl acetate/n-hexane VLC fraction of n-hexane extract, and the volatile oil of M. inconstrictum bulbs demonstrated the presence of phenolic monoterpenoid, fatty acid derivatives, and sesquiterpenoid structures as the main constituents, respectively.