1. Background
Acinetobacter is a genus of Gram-negative coccobacilli from the Moraxellaceae family. Acinetobacter species, including Acinetobacter baumannii, are opportunistic pathogens that can cause both community-acquired and nosocomial infections, especially in intensive care unit and high-dependency unit patients (1, 2). These bacteria have been isolated from various infections including ventilator-associated pneumonia, endocarditis, meningitis, skin and soft-tissue infections, urinary tract infection and prosthesis-related infections. A. baumannii has been isolated from various sources such as soil, water, animals and humans, and their presence in healthcare settings has made these microorganisms difficult to control (3, 4).
Until three decades ago, A. baumannii infections were treated effectively with conventional antibiotics (5, 6), but recently, due to the emergence of multi-drug resistant (MDR) strains, treatment of these infections has become challenging (7). Multi-drug resistant A. baumannii strains show resistance to at least four classes of antibiotics including penicillins, cephalosporins, fluoroquinolones, and aminoglycosides. Extensively drug-resistant (XDR) A. baumannii strains are resistant to the above antimicrobials and carbapenems as well. Pandrug-resistant (PDR) Acinetobacter strains are in fact XDR strains that are also resistant to polymyxins and tigecycline (8).
Compared to antibiotics, alternative antimicrobial agents such as medicinal plant extracts have fewer side effects and exhibit less toxicity. In addition, these agents could help control the spread of drug-resistant bacteria. Peganum harmala, also known as espand, is a perennial plant from the Zygophylacea family which is native to Asia and grows wild in the Middle East and parts of Southern Asia, especially India and Pakistan. Recently, the presence of alkaloids with antiviral properties in this plant and its seeds has attracted considerable attention (9).
2. Objectives
In this study, we compared antimicrobial activity of aqueous and ethanolic extracts of P. harmala and common antibiotics against A. baumannii strains isolated from patients with blood sepsis, burn wound infections and respiratory tract infections.
3. Methods
3.1. Research Population and Bacterial Isolation
This descriptive study was performed on 130 clinical samples collected from patients admitted to six hospitals of Golestan province (Iran) over a one-year period. The samples were taken from blood, burn wounds and trachea of patients after obtaining written consent and completion of a questionnaire. Blood samples were collected by trained nurses after disinfecting the skin of patients with 70% alcohol. Burn wound samples were collected using a cotton swab that was previously soaked in sterile physiological serum. Tracheal samples were taken by a specialist physician. Next, the samples were cultured on blood agar and examined for growth on MacConkey agar.
Gram-negative and lactose-negative bacteria were selected for further investigation. Acinetobacter strains were identified based on microbiological procedures including Gram-staining and indole, methyl red, Voges-Proskauer, motility, triple sugar iron agar, oxidative-fermentative with 10% glucose and DNase (for differentiation from Stenotrophomonas) tests. Acinetobacter baumannii species were identified using the following biochemical tests: lactose, galactose, glucose, mannose and rhamnose fermentation. A. baumannii ATCC 19606 was used as the positive control in all the experiments. Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923 were used as the positive control for the oxidase and the DNase tests, respectively.
3.2. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility was assessed using the disk diffusion agar (Kirby-Bauer) method. First, a bacterial suspension with the turbidity of 0.5 McFarland was prepared from fresh cultures of A. baumannii and later cultured on Muller-Hinton agar (Merck, Germany). Next, the following antibiotic disks (purchased from MAST Co., UK) were placed on the culture medium: levofloxacin (LVX5), doxycycline (D30), cefepime (CP30), amikacin (AN30), ticarcillin (TIC75), imipenem (IMP10), tigecycline (TGC15), rifampin (RA5), gentamicin (GM10), piperacillin (PIP100), ciprofloxacin (CIP5) and colistin (CS10). After 18 - 24 hours of culture at 37ºC, the diameter of growth inhibition zones was measured and results were interpreted as susceptible, intermediate and resistant according to the Clinical and Laboratory Standards Institute document M100-S25 (2015) (10).
3.3. Plant Collection and Preparation of P. harmala Extract
P. hamala seeds were collected from suburbs of Gorgan city, northern Iran, in April 2018. The seeds were washed, dried at room temperature and eventually powdered with an electric mill. Extraction was performed by maceration using ethanol as solvent. For this purpose, 25 g of dried seeds of P. harmala were mixed with equal amounts of heated 70% ethanol. The mixture was placed on a shaker for 3 to 4 days at room temperature. The resulting solution was filtered with a filter paper (Whatman No. 2, USA). Finally, the extract was concentrated in a bain-marie and then separated from the solvent using a rotary evaporator equipped with a vacuum pump (Edwards High Vacuum International Crawley, Sussex, England). Before complete drying, the extract was placed in the bain-marie for 24 hours to obtain a dry extract.
To prepare the aqueous extract, 25 g of milled seeds was soaked in 250 mL of sterile distilled water for three days. The solution was filtered and the solvent was evaporated under a fume hood, and dry weight was measured and freeze-dried.
3.4. Determining Minimal Inhibitory Concentration
After preparing a stock solution from the extract with 1% dimethyl sulfoxide (DMSO), minimal inhibitory concentration (MIC) of P. harmala extract at a range of 8 - 4096 μg/mL was determined by the broth microdilution method. Serial dilutions were made by adding 50λ of the extract to wells of a 96-well plate containing 50λ Muller-Hinton broth. Then, 50λ of antibiotic-resistant A. baumannii suspension (with turbidity of 0.5 McFarland) was inoculated onto each well. After overnight incubation at 37ºC, the growth rate was measured and compared with that of the positive (containing no antibiotic) and negative (without bacteria) controls.
3.5. Evaluation of the Antibacterial Effect of Extracts by Agar Well Diffusion
In order to investigate antimicrobial activity of the aqueous and ethanolic extracts of P. harmala against resistant A. baumannii strains, a suspension of antibiotic-resistant isolates with the turbidity of 0.5 McFarland (1.5 × 108 CFU/mL) was prepared and cultured on Muller-Hinton agar. Then, 100λ of different concentrations (500 - 31.25 mg/mL) of each extract was separately inoculated onto wells that were cut into the agar. A well containing distilled water was considered as negative control. After incubation, the results were reported based on the presence or absence of growth inhibition zones. An inhibition zone diameter of ≥ 15 mm indicated susceptibility and the diameter of ≤ 10 mm indicated resistance to the extract.
3.6. Identification of Components of the P. harmala Extract with Gas Chromatography-Mass Spectrometry Analysis
For this purpose, after injecting the extracts into GC columns under optimal conditions, the extracts were diluted with dichloromethane and then injected into a gas chromatography-mass spectrometry (GC-MS) instrument (Agilent GC MS, USA). Mass spectra and chromatograms were obtained and components of the extracts were identified and assessed both quantitatively and qualitatively.
3.7. Data Analysis
All the statistical analyses were performed with SPSS version 23.0. Chi-squared distribution was used to test for independence using the null-hypothesis (H0). Data were analyzed using the Kolmogorov-Smirnov test, Kruskal-Wallis test, and analysis of variance (ANOVA) at a significance level of 0.05.
4. Results
4.1. Demographic Specifications of Bacterial Isolates
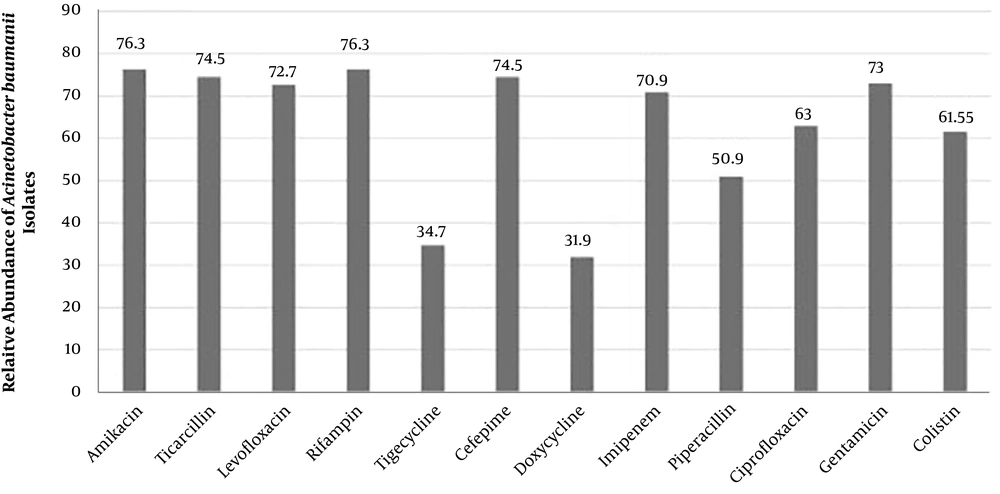
Overall, 55% of the isolates were identified as A. baumannii, which were mostly (67%) isolated from blood samples, male patients (76.4%) and patients aged 50 - 60 years (60%). The frequencies of XDR, PDR and MDR A. baumannii isolates were 58.2%, 32.7% and 61%, respectively. Moreover, the highest and lowest resistance rates were against amikacin and rifampin (both 76.3%) and doxycycline (31.9 %), respectively (Figure 1).
4.2. Determining Susceptibility to the Extracts Using the Broth Microdilution Method
Investigation of the effects of different concentrations (8 - 4096 μg/mL) of the extracts on the growth of MDR Acinetobacter isolates showed that both extracts were able to inhibit bacterial growth in a dose-dependent manner. The lowest concentration of the aqueous extract that inhibited the growth of 90% of the isolates (MIC90) was 1024 μg/mL, which was 4-fold lower than that of the ethanolic extract (4096 μg/mL). Moreover, the minimum concentration of the aqueous extract that inhibited the growth of 50% of the isolates (MIC50) was 256 μg/mL, which was significantly lower than that of the ethanolic extract (2048 μg/mL).
4.3. Antibacterial Effect of Extracts on Isolates
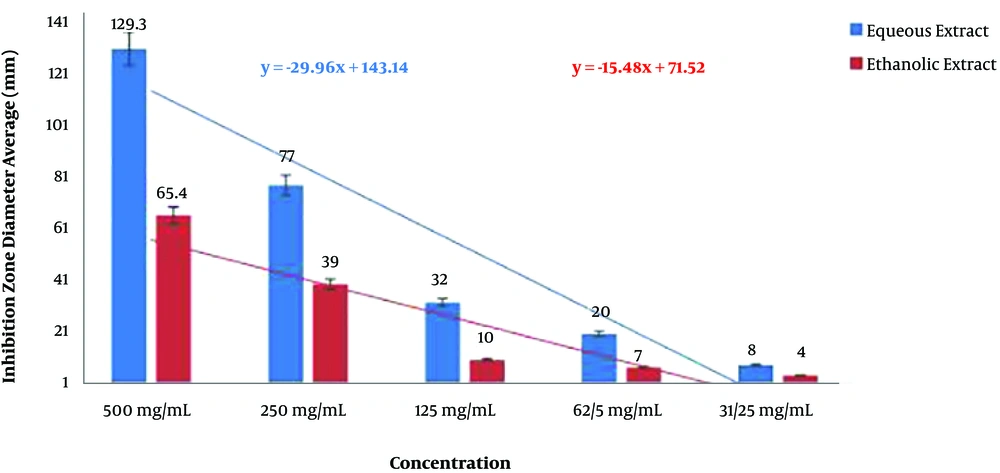
In the agar well diffusion test, both the aqueous and ethanolic extracts of P. harmala exhibited significant inhibitory effects on A. baumannii isolates (P < 0.05). Nevertheless, comparison of the mean diameter of inhibition zones for the two extracts showed that the inhibitory effects of the aqueous extract against A. baumannii isolates was significantly higher than that of the ethanolic extract (P < 0.05; Figure 2).
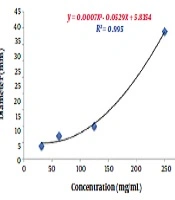
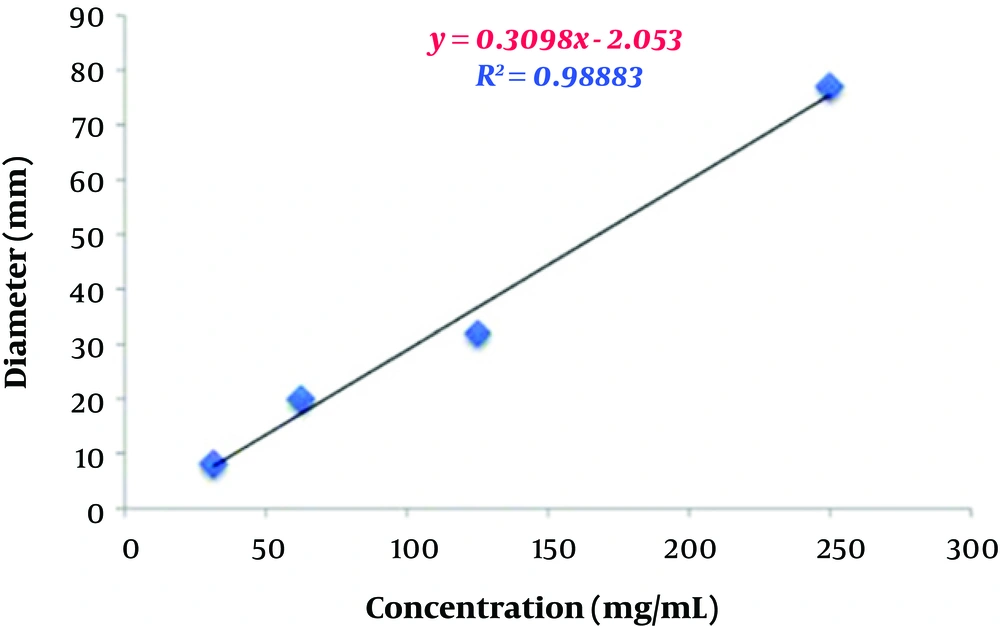
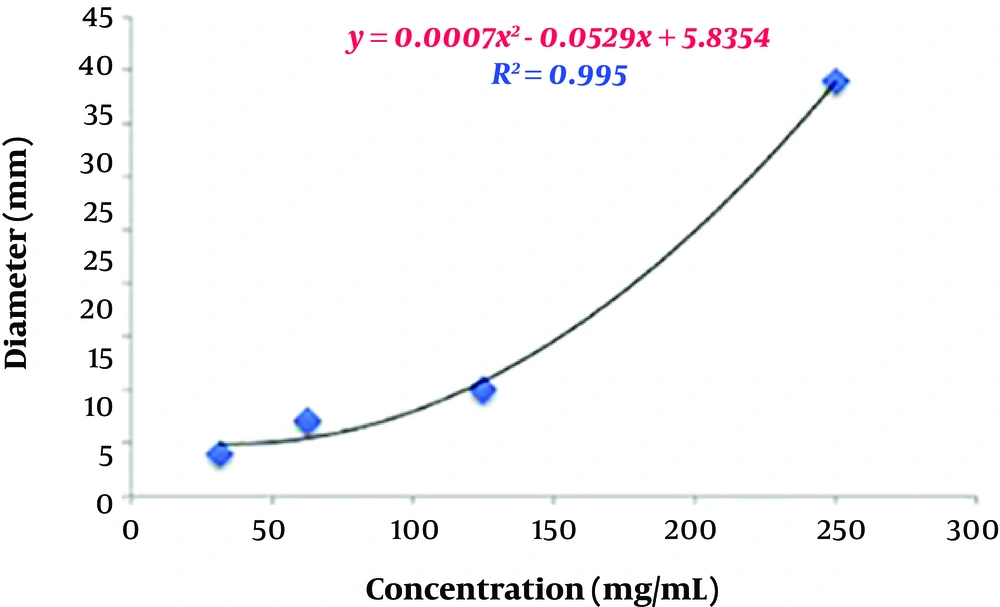
To estimate the relative concentration of the extracts, first, four concentrations and their respective diameters of growth inhibition zones were used to determine their regression equations and plot curves (Figures 3 and 4).
4.4. Comparison of the Antibacterial Effects of Aqueous and Ethanolic Extracts
The correlation coefficients between concentrations and inhibition zone diameters of the aqueous and ethanolic extracts were 0.98 mm and 0.99 mm, respectively. This indicates a high correlation between the concentration and inhibition zone diameters in the two groups. According to the regression analysis, the regression equations obtained from the data related to various concentrations of the aqueous and ethanolic extracts were y = 0.3098x - 2.053 and y = 0.0007x2 - 0.0529x + 5.8354, respectively. These two equations were used to predict the relative concentration of the aqueous and ethanolic extracts of P. harmala. Based on the calculations, the concentrations of the aqueous and ethanolic extracts were 244.66 mg/mL and 322.02 mg/mL, respectively. In other words, the concentration of the aqueous extract was about 1.23 times higher than that of the ethanolic extract. This higher yield also explains the higher inhibitory activity of the aqueous extract against A. baumannii isolates compared to the ethanolic extract.
4.5. Identification of the Constituents of P. harmala Extracts with the GC-MS Technique
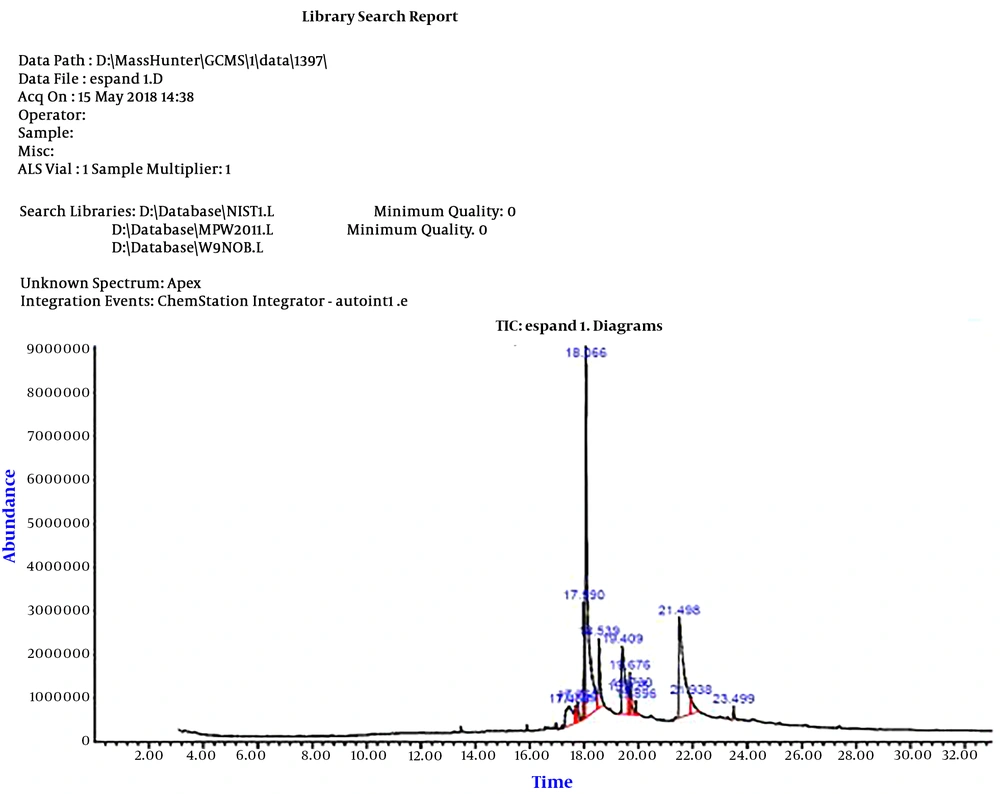
After analyzing the constituents of the aqueous extract, frequency and probable presence of some compounds in the extract were reported (Figure 5). The data obtained from the GC-MS analysis were further investigated by calculating the level of water solubility for each compound using the SwissADME server.
Based on the calculations, both vasicine/peganine (with a probability of 94%) and 8-hydroxy deoxy peganine (with a probability of 86%) constituted 40% of the compounds in the aqueous extract. In addition, with a probability of 98%, 6-Methoxy-9H-pyrido[3,4-b]indole andZharmine each constituted 23.45% of the aqueous extract.
The calculations also showed that the higher efficiency of the aqueous extract compared to the ethanolic extract is related to the degree of solubility of its components in water. In fact, the relatively higher solubility of these compounds in water than in ethanol results in the better extraction of these compounds in water.
Based on the results of the GC-MS analysis, vasicine/peganine and 8-hydroxy deoxy peganine can be categorized as soluble, while 6-Methoxy-9H-pyrido[3,4-b]indole and harmine are relatively soluble.
5. Discussion
The Acinetobacter genus is a major cause of nosocomial infections. In recent years, the number of Acinetobacter isolates from blood cultures has increased (11). In the present study, 67% of A. baumannii strains were isolated from blood samples, which is similar to the reports of a study performed in central of Iran (11).
Antibiotic resistance patterns among hospital pathogens may vary widely in different parts of a country or from one country to another. According to previous studies, first-line antibiotics for the treatment of A. baumannii infection include amikacin, carbapenems (imipenem, meropenem and doripenem), ceftazidime and quinolones (12). In addition, most pathogens have become almost completely resistant to some of the new antibiotics, such as broad-spectrum cephalosporins (cefotaxime and ceftazidime). Imipenem is recognized as the most effective drug against the infections caused by Acinetobacter spp., but recent evidence has indicated the spread of imipenem-resistant strains. The highest rate of resistance to imipenem among Acinetobacter spp. has been observed in A. baumannii. The widespread emergence of resistance to imipenem is a serious threat to the future of healthcare. The extent of antimicrobial resistance varies across countries and is influenced by environmental factors and the application of different antimicrobials (13, 14).
In this study, A. baumannii isolates were resistant to all the tested antibiotics, and the highest rate of resistance was observed against amikacin and rifampin (76.3%). Following these two antibiotics, the highest resistance rates were against ticarcillin and cefepime (74.5%), gentamicin (73%), levofloxacin (72.7%) and imipenem (70.9%). The lowest resistance rates were against tigecycline (34.7%) and doxycycline (31.9%).
Similar to our findings in a previous study performed in Iran, more than 60% and 78% of the MDR bacteria were resistance to amikacin (11) and imipenem (15), while in another study the rates of imipenem and amikacin resistance were 58% and 40% among Acinetobacter strains, respectively (16).
In other studies, the rates of resistance to amikacin among Acinetobacter spp. were 47.2% (17) and 85.2% (12). This difference could be due to the extensive use of antibiotics in different hospital wards and the uncontrolled and arbitrary use of these drugs in our study area. Furthermore, the quality of the antibiotic disks and the techniques used for determining antibiotic susceptibility in microbiological laboratories could have affected the results. The emergence of MDR A. baumannii strains has become a growing global health problem (18, 19). In this regard, previous studies in Iran reported the frequency of MDR A. baumannii strains to be approximately 80% (11).
The high prevalence of MDR Acinetobacter strains has been also found in other countries (20, 21). Inconsistencies between findings of studies may be attributed to the differences in the type of studied clinical samples, time of the study and the therapeutic strategies, including the use of broad-spectrum antibiotics in initial therapy. Colistin is known as an effective antibiotic against MDR A. baumannii strains because of its broad-spectrum antimicrobial activity against Gram-negative bacteria. However, an increase in the prevalence of colistin-resistant isolates has been noted in recent years (18).
Given the results obtained from our study, the use of different antimicrobial compounds seems sensible for controlling such resistances. One of these compounds is the P. harmala extract that contains various alkaloids with antimicrobial effects and no side effects (22, 23). A previous study also demonstrated the favorable antimicrobial activity of this extract against various species of bacteria, particularly drug-resistant ones (24).
In our study, 87.3% and 50% of the Acinetobacter isolates were susceptible to the aqueous and ethanolic extracts of P. harmala, respectively. In other words, the aqueous extract of P. harmala exhibited significantly higher antimicrobial activity against Acinetobacter isolates compared to the ethanolic extract. Contrary to this finding, researchers have reported that the aqueous extract of P. harmala is significantly more effective against Lactobacillus and Candida spp. compared to the aqueous extract (25).
Our results also showed that the type of solvent had a significant impact on the extraction of active compounds from the plant. Polar solvents such as ethanol and water are thought to be more suitable for the extraction of bioactive secondary metabolites of plants. The antibacterial activity of the ethanolic extract of P. harmala can be attributed to the presence of alkaloids such as harmine and harmaline. The abundance of these compounds in the aqueous extract may also explain the higher antimicrobial activity (23).
In the microdilution method, the antibacterial activity of the aqueous extract increased in a dose-dependent manner, such that 2048 μg/mL of the extract was able to inhibit the growth of almost all resistant Acinetobacter isolates.
In the present study, the number of susceptible isolates to the aqueous extract was greater than that of resistant ones, which indicates the significant inhibitory effect of the aqueous extract of P. harmala against A. baumannii isolates.
5.1. Conclusions
We observed that the prevalence of MDR A. baumannii isolates is high in blood samples of patients with sepsis, which highlights the importance of this pathogen in developing nosocomial infections and a need for programs to control the spread of this pathogen.
The aqueous extract of P. harmala has favorable antimicrobial activity against antibiotic-resistant A. baumannii isolates. This extract has low toxicity and great antimicrobial activity, making it a cost-effective alternative to chemical antibiotics for the treatment of infections caused by resistant Acinetobacter strains. Based on the results of GC-MS analysis, the higher antibacterial activity of the aqueous extract compared to the ethanolic extract could be related to the increased concentration of vasicine/peganine and 8-hydroxy deoxy peganine in the aqueous extract, which might be due to their high water solubility. Therefore, it is recommended to conduct further research on the pharmaceutical potential and antibacterial activity of these compounds.