1. Background
Direct human contact with some animals has caused a dramatic increase in zoonotic diseases. Hydatidosis occurs as a result of human and herbivorous infections with the larval phase of Echinococcus granulosus. The disease develops due to contact with dogs (final host) and livestock (intermediate host) in animal husbandry centers; therefore, humans are also at risk of this disease (1). The importance of this disease is linked to the involvement of sensitive organs, such as the liver and lungs. Studies have shown that the occurrence of hydatid cyst is 1 per 100,000 people in Iran. The rate of infection with Echinococcus granulosus parasites in the canine is 45 - 63% in Mazandaran, West Azerbaijan, Isfahan, and Ilam provinces and approximately 20% in other regions of Iran (1). Hydatidosis may be diagnosed many years after infection. Surgical treatment has been always challenging, since during surgery, there is a risk of cyst rupture, release of hydatid fluid, migration of protoscolices to other tissues, and relapse of the disease (2, 3). Considering the possible occurrence of secondary hydatid cysts during surgery (10 - 30%), scolicidal agents are used to kill enterocytic protoscolices during surgery (2). Until now, many compounds have been used as scolicidal agents. However, finding more effective agents with fewer complications seems necessary. Accordingly, attention to plant-derived extracts has increased, because these substances have few side effects and can be used as food in many cases (4).
In Iran, various plant derivatives, such as Berberis vulgaris, Ephedra major, pomegranate root extract, Peganum harmala, and vinegar, have been investigated for their effects on hydatid cyst protoscolices (5-9). Allium is one of the main members of Amaryllidaceae family. This family consists of nearly 850 species throughout the Northern Hemisphere (10). Some of these species are used in traditional drugs to treat headaches, intestinal worms, cholera, and dysentery. Several investigations have shown the antifungal and antibacterial properties of Allium (6). Allium noeanum is a native plant grown in Markazi and Lorestan provinces of Iran, known as “Bonsor”, as well as Kermanshah and Kohgiluyeh and Boyerahmad provinces, where it is known as “Sarodak” or “Valk Suri”. This plant has been used in traditional medicine for treating certain diseases, but no detailed study has yet determined its properties. Generally, it is not difficult for researchers to have access to A. noeanum, since it is a native and self-growing plant, both easy and inexpensive to collect.
Apoptosis is a form of programmed cell death that removes old and harmful cells under normal conditions (11). It is controlled and regulated by some caspases, including caspase-3 (12). Caspase-3 is an endoprotease, which plays a central role in the execution phase of apoptosis (13). Since apoptosis is a process of cellular self-destruction, it provides a convenient way to eliminate parasitic agents, such as Echinococcus protoscolices (14). Some studies have shown that herbal extracts can induce the apoptotic pathway (14).
2. Objectives
Since the anti-parasitic properties of A. noeanum have not been examined in the literature, in the current research, we aimed to evaluate these properties and to determine the effects of crude and flavonoid extracts of this plant on protoscolices and apoptosis in hydatid cyst walls.
3. Methods
3.1. Preparation of Protoscolices and Hydatid Tissue
The hydatid liver cysts of sheep were obtained from a slaughterhouse in Arak and transferred to the parasitology laboratory of Arak University of Medical Sciences. The contents of the cysts were drained by a sterile syringe, shed in a sterile glass cylinder, and settled for 30 minutes. Next, the supernatant was discarded, and the precipitate was rinsed three times with phosphate buffer (pH = 7.2). The mortality rate of protoscolices was estimated by using 0.1% eosin stain and studying the activity of flame cells under an optical microscope. Live protoscolices were colorless, while dead protoscolices were red. Finally, a suspension of 9,000 to 10,000 protoscolices per milliliter was prepared. The suspension, with at least 90% of protoscolices being alive, was transferred to a dark container and kept at 4°C (5). After the evacuation of hydatid cysts, cystic layers, consisting of a laminate layer and a germinal layer, were isolated from the host tissue (sheep). The layers were rinsed with phosphate buffer and used for histological studies.
3.2. Herbal Extracts Preparation
The mature and fresh aerial part of A. noeanum (CMG1) was collected from Shazand in Markazi Province, Iran (CMG1 = Mehrdad Godarzi Collection Number in Arak University Herbarium, not listed in herbarium index). Samples were authenticated by taxonomist Dr. Mitra Noori based on available references (15, 16). The voucher specimen was deposited at the Department of Biology, Faculty of Science, Arak University, Iran (CMG1, 07 June 2016). The leaves of plant were then washed, air-dried, and milled. Next, 300 g of the sample powder was added to a container with 3000 mL of ethanol (70% V/V) and kept for three consecutive days. Finally, the extract was placed in a rotary machine for two days to be concentrated, and the extract was divided into two portions. One portion was used as the crude extract, and the other portion was used for preparing the flavonoid extract.
To identifying of flavonoids compounds and their groups in crude extract was used paper chromatography. Then, the flavonoid extract was prepared using the acid hydrolysis method. For this purpose, 2 M hydrochloric acid was added to the crude extract and then placed in a water bath at 100°C for 30 minutes. In order to isolate the flavonoid extract from the non-flavonoid fraction, 200 mL of ethyl acetate was added to the solution. Finally, the flavonoid extract was dried using Heidolph Rotary evaporator Laborota and dissolved in MeOH 80% (17).
To identifying specific flavonoid compounds of A. noeanum co-chromatography with standards was performed. For this purpose, the flavonoid content (gram per kg plant dry weight, g/kg DW) was measured using the weight/volume (W/V) method after dissolving the dry extract in 70% (V/V) ethanol. Flavonoid standards available for comparison during the study included apigenin, chrysin, genistein, hesperidin, isorhamnetin, kaempferol, luteolin, morin, myricetin, naringenin, quercetin, rhamnetin, rutin, tricine, and vitexin, all obtained commercially (rutin from Merck, apigenin and luteolin from Sigma, and the rest from Fluka). Then, cellulose sheet TLC plate were used to help flavonoid identification after running in butanol-acetic acid-water (BAW), chloroform-acetic acid-water (CAW), and Ferrostaal solvents. The TLC plate was viewed under UV 245 nm, and the ratio to front (Rf) values and color of each spot were compared with the standards. The flavonoid extract was kept in dark vials and stored in cool conditions until further use (18, 19).
Finally, the amount of active ingredient in the crude and flavonoid extracts of the A. noeanum was 0.49 and 1.35 gr/mL, respectively.
3.3. Experiment Design
3.3.1. Effects of Herbal Extracts on Protoscolices In Vitro
Crude extract was prepared at concentrations of 100% (0.49 gr/mL), 75% (0.367 gr/mL), 50% (0.245 gr/mL), and 25% (0.122 gr/mL). Flavonoid extract was prepared at concentrations of 100% (1.35 gr/mL), 75% (1.012 gr/mL), 50% (0.675 gr/mL), and 25% (0.337 gr/mL).
Next, 100 μL of protoscolices suspension was exposed to an equal volume of each extract for 30 seconds and 1, 5, 10, 20, and 30 minutes at ambient temperature. At the end of each interval, the number of dead protoscolices was counted in 100 protoscolices; this measurement was carried out separately for each of the four concentrations of the extracts. The mortality rate of protoscolices in phosphate buffer (negative control) and 10% formalin (positive control) was also determined. The tests were repeated three times.
3.3.2. Effect of Herbal Extracts on Hydatid Cyst Tissue In Vitro
A number of hydatid cyst wall sections were placed in control plates, containing phosphate buffer. Other sections were transferred to the plates, containing 100% concentrations of both herbal extracts (separately), and kept for 24 hours in a CO2 incubator at 37°C; this was performed to assess the effect of extracts on the hydatid cyst wall. Then, each tissue was located in a container with formalin. Tissue preparation included fixation, preparation of paraffin blocks, tissue cutting, and hematoxylin-eosin staining on each tissue (20).
3.3.3. Immunohistochemistry for Caspase-3 Detection
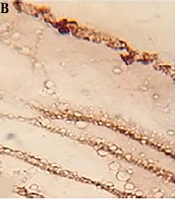
Immunohistochemistry was performed using a rabbit polyclonal antibody (AB4751, Abcam Corporation). Caspase-3 antibodies were diluted at 1:300. All sections were placed at high temperature for antigen retrieval, and the staining process was carried out based on the kit manufacturer’s instructions. Finally, all the sections were placed in DAB to be counted in hematoxylin and observed by light microscopy. Brownish spots in the cytoplasm were considered as caspase-3-positive. To exclude false positive responses, slides stained with PBS, replacing the primary antibodies, were used as the controls (13).
3.4. Statistical Analysis
Data were analyzed in Excel (2019) and SPSS (ver.16). Kolmogorov-Smirnov test was used to examine the normality of data. Data were presented as the mean values of three separate experiments and expressed as mean ± SD. Differences between the subgroups and the control group were analyzed with repeated measures test and Tukey’s honest significant difference (HSD) test. Statistical significance was considered to be P < 0.05.
4. Results
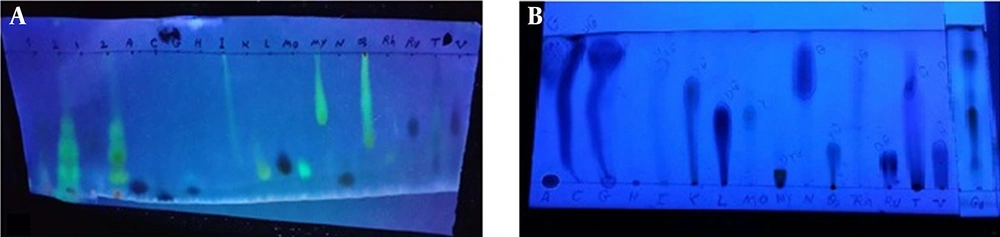
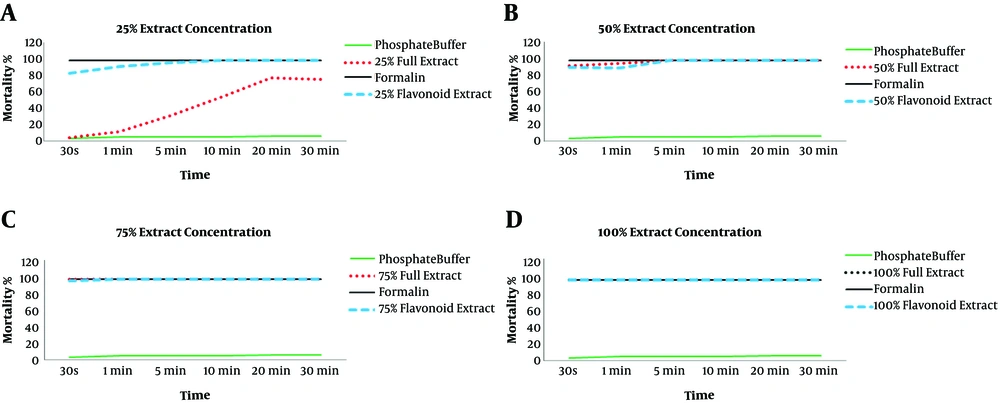
The chromatographic results showed that the phytochemical compounds of A. noeanum leaf contained flavonoid sulfates and no aglycones. The flavonoid components of this plant included apigenin, isorhamnetin, luteolin, kaempferol, myricetin, quercetin, rutin, morin, rhamnetin, and vitexin (Figure 1). Qualitative investigation of A. noeanum extracts showed that they contained 472.7 g/kg DW of effective material and 86 g/kg DW of flavonoids. The average mortality of protoscolices in exposure to different concentrations of crude extract and flavonoid extracts is shown in Table 1 and Table 2, respectively. The lowest concentration of the crude extract of A. noeanum (25%), even at maximum exposure time (30 minutes), could not kill all parasites, but higher concentrations of this extract destroyed all the parasites (Table 1).
The result of chromatography. A) A. noeanum flavonoids extract chromatogram using descending paper chromatography in BAW solvent, B) A. noeanum flavonoids extract chromatogram using ascending thin layer chromatography in CAW solvent. Abbreviations: A (apigenin), C (chrysin), G (genistein), H (hesperidin), I (isorhamnetin), K (kaempferol), L (luteolin), Mo (morin), My (myricetin), N (naringenin), Q (quercetin), Rh (rhamnetin), Ru (rutin), T (tricine), and V (vitexin); G1 (1)= A. noeanum crude extract, G2 (2)= A. noeanum acid hydrolysis flavonoids extract.
| Mortality Over Time | Mortality (% ± SD) | |||||
|---|---|---|---|---|---|---|
| 30 Sec | 1 Min | 5 Min | 10 Min | 20 Min | 30 Min | |
| Extract concentrations (gr/mL) | ||||||
| 25% (0.122) | 4.7 ± 0.6 | 12 ± 2.6 | 31.7 ± 3 | 53.7± 5.9 | 78.7 ± 5.9 | 76.7 ± 3 |
| 50% (0.245) | 93.3 ± 1.5 | 95.7 ± 2.1 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 75% (0.367) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 100% (0.49) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Negative control (buffer) | 4 ± 1 | 5 ± 1.7 | 5 ± 0 | 5 ± 2 | 6 ± 1 | 6 ± 0 |
| Positive control (formalin) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
All concentrations of the flavonoid extract of A. noeanum led to the death of 100% of parasites, while increasing the concentration of the flavonoid extract reduced the length of time to death (Table 2).
| Mortality Over Time | Mortality (% ± SD) | |||||
|---|---|---|---|---|---|---|
| 30 Sec | 1 Min | 5 Min | 10 Min | 20 Min | 30 Min | |
| Extract concentrations (gr/mL) | ||||||
| 25% (0.337) | 83.7 ± 4.7 | 92.3 ± 2.9 | 97 ± 1.7 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 50% (0.675) | 91.7 ± 2.9 | 90.3 ± 2.5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 75% (1.012) | 97.7 0.6± 0.6 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 100% (1.35) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Negative control (buffer) | 4 ± 1 | 5 ± 1.7 | 5 ± 0 | 5 ± 2 | 6 ± 1 | 6 ± 0 |
| Positive control (formalin) | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
Comparison of mortality rate of protoscolices in exposure to different concentration of crude extract and flavonoid extracts of A. Noeanum at different exposure times was shown in Figure 2.
Comparison of the mortality rate of protoscolices in exposure to 25% (0.122 gr/mL), 50% (0.245 gr/mL), 75% (0.367 gr/mL) and 100% (0.49 gr/ml) concentrations of crude extract with mortality rate of the parasite in exposure to 25% (0.337 gr/mL), 50% (0.675 gr/mL), 75% (1.012 gr/mL) and 100% (1.35 gr/mL) concentrations of flavonoid extract
Statistical comparison of protoscolices mortality showed three effective factors, including the type of herbal extract, amount of extract, and exposure time, in the rate of protoscolices mortality, and a statistically significant difference was observed (P < 0.001).
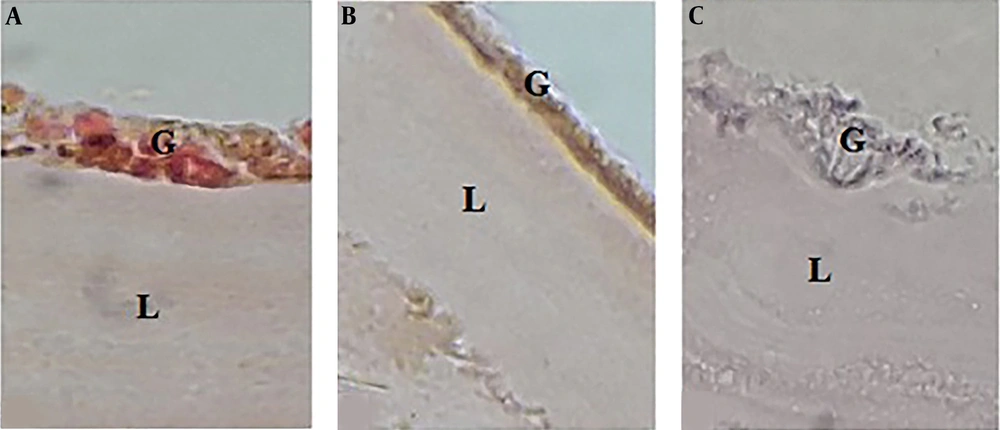
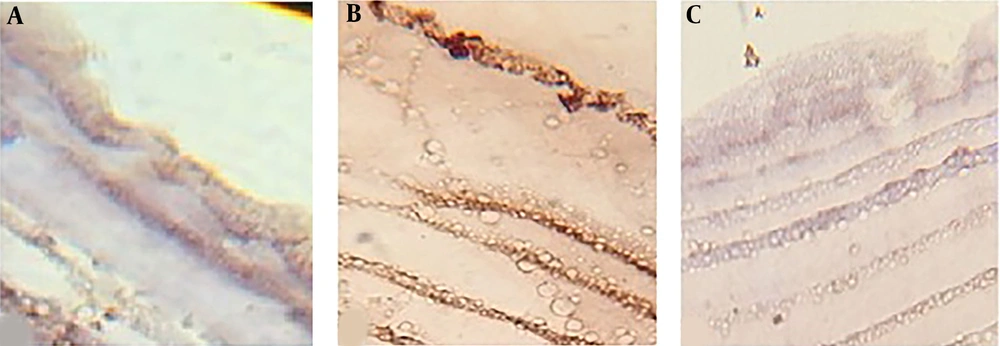
The effect of A. noeanum extract on the hydatid cyst wall showed that in the control group, where the cyst wall was exposed to a buffer solution, the laminated layer was uniform and clear. Also, the structure of germinal layer was clear, and its nuclei were visible. By exposing the cyst wall to the crude extract, the laminated layer was found to have curved, rough, and incrassated veins, and the structure of germinal layer was unclear, and its nuclei had disappeared. Moreover, by exposing the cyst wall to the flavonoid extract, the laminated layer was found to have incrassated, rough, and curved veins, and the laminated layer was unclear, and its nuclei had disappeared (Figure 3).
Histological changes in the hydatid cyst wall. A) After exposing the cyst to phosphate buffer solution, the germinal layer and its nuclei were clear, and the laminated layer was uniform and clear. B) After exposing the cyst to 100% concentration (0.49 gr/mL) of crude extract herbal extract, the germinal layer was found to be unclear, and its nuclei had disappeared. The laminated layer was also uneven and rough. C) By exposing the cyst to 100% concentration (1.35 gr/mL) of the flavonoid extract, the germinal layer was found to be completely destroyed, and the laminated layer was rough, curved, and uneven.
Study of caspase-3 activity in the hydatid cyst wall by immunohistochemistry showed that caspase-3 activity in the cyst walls exposed to flavonoids was less than the crude extract of A. noeanum. The results showed that the activity of caspase-3 in the cyst walls exposed to both types of the extract was more comparable to that of the control group (Figure 4).
5. Discussion
Resistance and side effects of some drugs have led researchers to seek new effective compounds, such as herbal derivatives to treat infectious diseases (21). The mechanism of the effects of plant derivatives on microorganisms depends on their composition (22). Allium plants are widely cultivated in the world and are used as food and herbal medicine. The antibacterial properties of some species of this herb have been confirmed in the literature (23). Recent research has also shown that Allium derivatives can be used as active edible coating (24).
Some compounds of Allium contain fatty acids, phospholipids, and amino acids (25). A. noeanum is a new species of this genus, which has not been studied so far. This plant is native to Markazi Province and some neighboring provinces. Although, only it is grown a few weeks during spring and it is possible to gather it during this time. In the current study, two types of extracts (crude and flavonoid) from a specific Allium species were used. According to the present results, concentrations above 50% of the crude extract of A. noeanum resulted in the death of all protoscolices in 30 seconds; however, concentrations below 50% could not destroy protoscolices, even within 30 minutes. In contrast, all concentrations of the flavonoid extract of A. noeanum could kill all protoscolices after 10 minutes. The mortality rate, however, depends on the concentration of the extract and duration of exposure. Comparison of the mortality rate of parasites in exposure to crude extract and flavonoid extracts showed that the amount and duration of exposure significantly affected the mortality rate (P < 0.001).
Despite the approved scolicidal properties of herbal substances, the mechanism of their activity remains unclear. Apoptosis is one of the possible mechanisms of hydatid cyst suppression among natural immune pathways (26). According to the evaluation of tissue changes induced by A. noeanum extracts in the hydatid cyst wall, although the cyst wall structure was most damaged in exposure to the flavonoid extract, apoptotic activity was higher in the cystic wall exposed to the crude extract. Overall, several investigations have been performed on the scolicidal effects of different herbal extracts. Although the mechanism of scolicidal activity is not specified, some species, such as Nigella sativa, can inhibit histone deacetylase enzymes and DNA synthesis, while some others, such as Myrtus communis, can increase the activity of caspase-3 and caspase-9 (24, 26).
The current study is the first research on the therapeutic effects of A. noeanum. Since deactivation of the germinal layer and protoscolices of hydatid cyst is the main goal of therapeutic methods, this study investigated the mortality rate of protoscolices, as well as structural and apoptotic changes in the hydatid cyst wall. It was found that A. noeanum extracts lead to apoptosis in the cystic wall, which is similar to some previous studies (13).
Flavonoids are polyphenolic compounds, which are mostly found in plants and appear to be strong antioxidants and anti-radical agents (27, 28). Previously, the scolicidal activity of propolis was confirmed. According to the available evidence, researchers attribute the scolicidal effect of propolis to flavonoid compounds because of their antimicrobial properties (29). Additionally, the influence of flavonoid compounds on some parasites, including Leishmania, has been confirmed (30). Use of herbal medicines has been advocated in developing countries, as they are nutritious and compatible with the human body and have minor side effects. Since flavonoids are different in nature, their effects may vary. In future studies, it is suggested to isolate the flavonoids of A. noeanum extracts in order to determine their effective components and to evaluate the mechanism and extent of their effects on protoscolices. Moreover, it is necessary to study the effect of these extracts on the host tissues (animals or humans).
5.1. Conclusions
In the current study, the scolicidal activity of crude extract and flavonoid extracts of A. noeanum against protoscolices was confirmed. Moreover, the dose- and time-dependent scolicidal activity of the extracts was approved. It was found that the apoptotic effect of the crude extract of A. noeanum was greater than that of the flavonoid extract; therefore, investigation of the scolicidal effects of other compounds in the extracts is recommended. This study is the first research on the anti-parasitic properties of A. noeanum. However, the ability of live protoscolices to produce hydatid cysts should be studied in vivo, as protoscolices exposed to Allium extracts may be alive, but lose the potential of hydatid cyst formation.