1. Background
Wound healing is a complex and well-orchestrated process of restoration of missing structures and the function of damaged tissue after injury (1). The wound healing process is divided into four phases, including hemostasis, inflammation, proliferation, and tissue remodeling (2). A cascade of cellular events, including the interaction of blood cells, inflammatory cells, proteins, proteases, growth factors, and extracellular matrix components, occurs within these phases (3). Many factors, including infection, foreign body, the paucity of blood supply, and poor nutritional and health status, may affect the wound healing process, thus causing impaired wound healing (4). Chronic, non-healing wounds can cause enormous suffering and disabilities. Despite advances in wound science, pharmacotherapy, and wound care practice, wound healing remains a challenge to many clinicians and a major and increasing threat to public health and the economy (5). During recent years, increasing attention has been directed toward studying the wound healing activity of natural substances due to their therapeutic activities, including antimicrobial, anti-inflammatory, and cell proliferative actions (6).
Cinnamic Acid (CA) is an organic compound that naturally occurs in many plants. Cinnamic acid and related derivatives, displaying a wide range of biological activities and low toxicity, are of significant interest for producing new effective and safe medications (7). During the last two decades, several studies have been performed on the medicinal application of cinnamic-related molecules, and the results indicated that CA derivatives possess anticancer (8, 9), antibacterial (7, 10, 11), antifungal (12), anti-inflammatory (13), and antiatherogenic (14) properties. Cinnamic acid is a compound of low toxicity for animals, humans, and the environment (15-17). Cinnamic acid and its related compounds are widely used in cosmetics and possess various functions such as UV protection and perfuming function (18). In this study, we aimed to investigate the effect of CA on the wound-healing process based on the assessment of the wound closure rate, hydroxyproline content of tissue, and histological state of the wound-healing process. In this study, we used rabbit because it is relatively inexpensive and well-suited to testing potential therapeutics (rabbit and human skin respond similarly to aging, delayed healing, and various topical drugs), and the animal has an appropriate size (19).
2. Objectives
Previous studies have shown that CA possesses anti-inflammatory and antioxidant activities. Thus, this study aimed to evaluate its healing activity on full-thickness wounds in animal models.
3. Methods
3.1. Preparation of Cinnamic Acid Ointment
Cinnamic acid was purchased from Merck Millipore (Germany) (CAS number 140-10-3). Then, 0.1, 1, and 10 g of CA were incorporated into 100 g of eucerin to prepare 0.1, 1, and 10% (W/W) CA ointments, respectively. The ointment was prepared by the levigation method. The physical stability test of the ointment was carried out for five weeks at different temperatures including 4°C, 25°C, and 35°C. The prepared ointment was found to be stable at different temperatures. The pH of the ointment was measured by using a digital pH meter during stability studies on the first and 35th days of ointment preparation.
3.2. Animals
Healthy white New Zealand rabbits of both sexes weighing 2,500-3,000 g were used in this study. The animals were purchased from the Pasteur Institute of Iran (Tehran, Iran). They were kept in the standard condition in an animal house at 26 ± 2°C, humidity of 45-55%, and 12-h light/dark cycles. Food and water were provided ad libitum. No animal was sacrificed in this study. The sample size for this study was calculated based on the three Rs, standing for Replacement, Reduction, and Refinement. The work is reported following the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments) (20). The animal protocol was approved by the Ethics Committee of Hamadan University of Medical Sciences.
3.3. Experimental Procedure
We randomly divided 36 rabbits into six equal groups. Two identical full-thickness excisional wounds (20 × 20 mm) were created on the back of each animal (21). Group 1 received no treatment (negative control), group 2 was treated with eucerin (ointment vehicle), group 3 was treated with phenytoin 1% cream (positive control), and groups 4, 5, and 6 were treated with ointments containing 0.1, 1, and 10% (W/W) of CA in eucerin. Treatments were applied topically twice daily, from the day after wound creation until the complete healing of the wounds.
3.4. Wound Contraction Evaluation
The wounds were photographed every day. The wound surface area was calculated in the captured images using Image JR software (22). The wound-healing percentage on a specific day was calculated based on the reduction of the wound area surface as described below:
% Wound healing = wound surface area at day 1 – wound surface area at specific day/woundsurface area at day 1 × 100
3.5. Hydroxyproline Assay
The hydroxyproline content of the wound tissue sample obtained on days 7 and 14 after wounding was determined by using the method of Edwards and O'Brien (23). Briefly, tissue samples were hydrolyzed in 6N HCl for 4 h at 120°C. After the neutralization of hydrolysates, they were oxidized by Chloramine-T. By the addition of 0.4 M perchloric acid, the reaction was terminated, and the produced color with the Ehrlich reagent was read by an ELISA plate reader at 540 nm. The calculated hydroxyproline content of samples was expressed as mg/g of tissue weight.
3.6. Histopathological Study
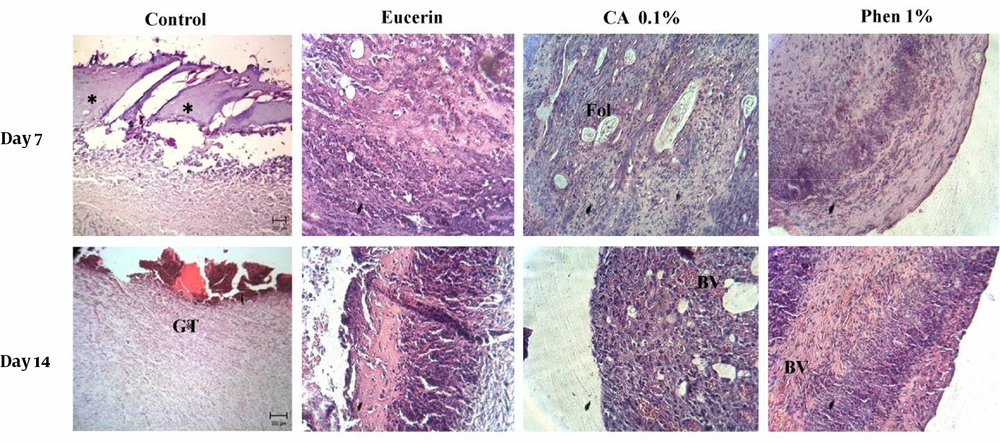
The wound tissue samples were taken for histopathological studies on days 7 and 14 post wounding. The wound site in each animal was anesthetized by lidocaine 2%, and a section of the healing tissue was cut. The samples were fixed in 10% buffered formalin, embedded in paraffin, sectioned to 5-µm thicknesses, and stained with hematoxylin and eosin for histopathological study. The histopathological assessment of wounds was performed based on the percentage of re-epithelialization, presence or absence of inflammatory cells, fibroblasts, and new vessels. The area of granulation tissue was also evaluated.
3.7. Statistical Analysis
The data were analyzed by using one-way ANOVA and Tukey post hoc tests. The P values of less than 0.05 were considered statistically significant. All tests were carried out using SPSS version 22 software (SPSS Inc., Chicago, USA).
4. Results
4.1. Wound Contraction
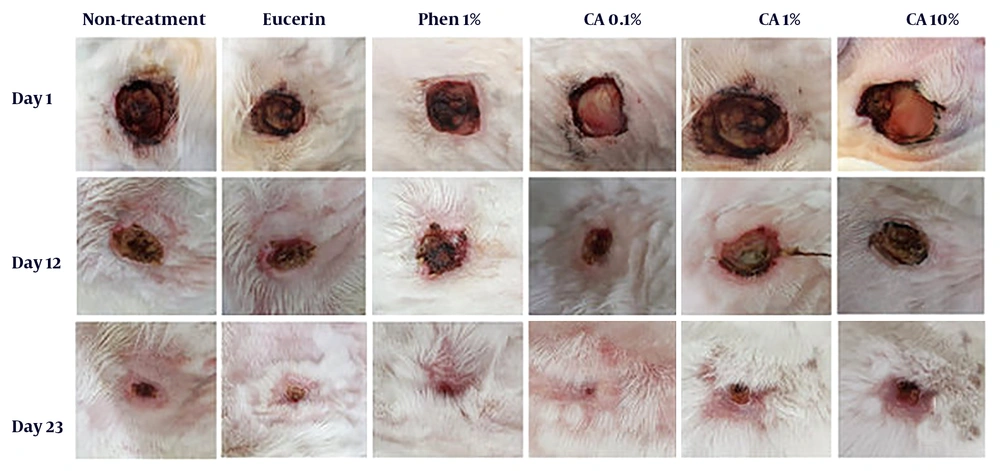
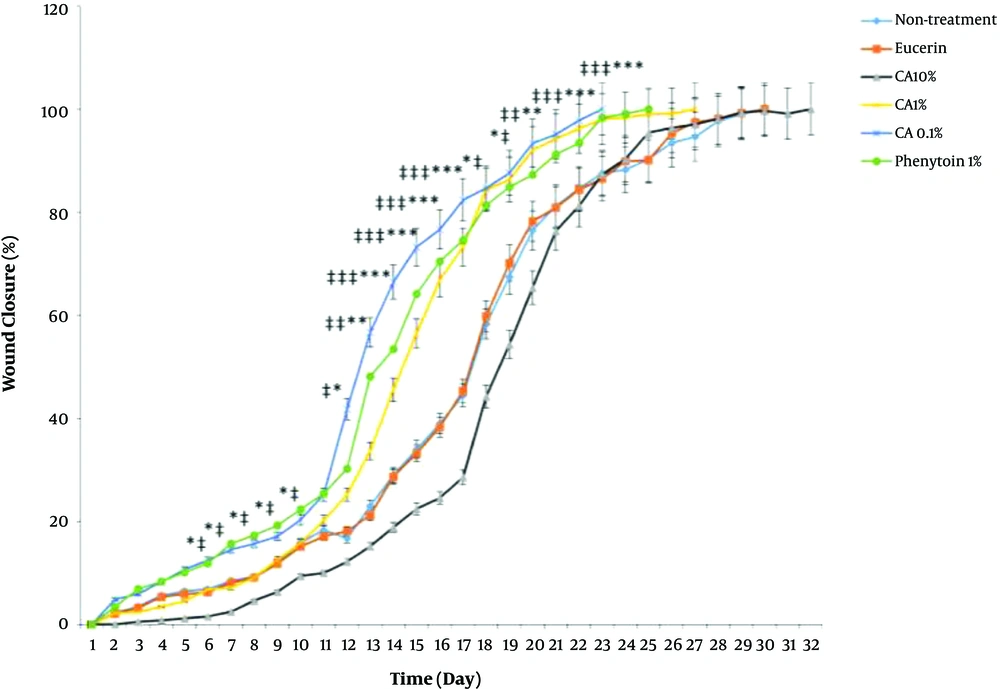
Complete wound closure in the shortest time (23 days) was observed in CA 0.1%-treated animals with a better healing pattern (Figure 1). The time to complete wound closure in animals treated with phenytoin cream, as a positive control, was about 25 days. The complete wound closure occurred in 27 days for CA 1% ointment, 30 days for no-treatment and eucerin-treated groups, and 32 days for CA 10% ointment-treated group. The times to complete wound closure in CA 0.1% and phenytoin 1%-treated groups were significantly shorter (P < 0.05) than those of no-treatment, eucerin, and CA 10%-treated groups. There were no significant differences between the no-treatment, eucerin, and CA 10%-treated groups in the wound closure rate and time to complete wound closure (Figure 2). The wound closure rate in CA 1%-treated group was significantly different (P < 0.05) from no treatment and eucerin-treated animals in few days.
Effect of Cinnamic acid on wound closure percentage in different days. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate the statistically significant differences between CA 0.1% group and no-treatment group, #P < 0.05, ##P < 0.01, and ###P < 0.001 indicate the statistically significant differences between CA 0.1% group and eucerin-treated group
4.2. Hydroxyproline Content in Tissue Samples
The hydroxyproline content of tissue samples in experimental groups was measured on days 7 and 14 post wounding. The hydroxyproline levels of tissue samples obtained from the CA 0.1%-treated group was significantly higher than that of no-treatment and eucerin-treated groups on days 7 and 14 after wound creation. The hydroxyproline content of samples obtained from animals treated with CA 1% or phenytoin cream was significantly higher than that of negative and vehicle control groups on day 14 of wounding (Table 1).
| Groups | day 7th | day 14th |
|---|---|---|
| No-treatment | 53.2 ± 1.35 | 58.7 ± 3.94 |
| Eucerin | 54.4 ± 1.23 | 55.4 ± 0.96 *# |
| Phenytoin | 69.6 ± 2.62 | 96.6 ± 3.67 |
| CA 0.1% | 74.8 ± 1.00 *# | 114.1 ± 2.45 **## |
| CA 1% | 69.3 ± 0.76 | 88.4 ± 0.92 *# |
| CA 10% | 56.0 ± 1.36 | 70.3 ± 2.8 |
Abbreviation: CA, cinnamic acid.
aData are expressed as Mean ± SEM. * P < 0.05 and ** P < 0.01 indicate the significant difference from no-treatment group. # P < 0.05 and ## P < 0.01 significant differences from eucerin treated group.
4.3. Histopathological Study
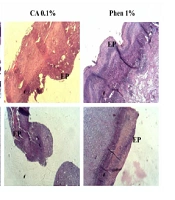
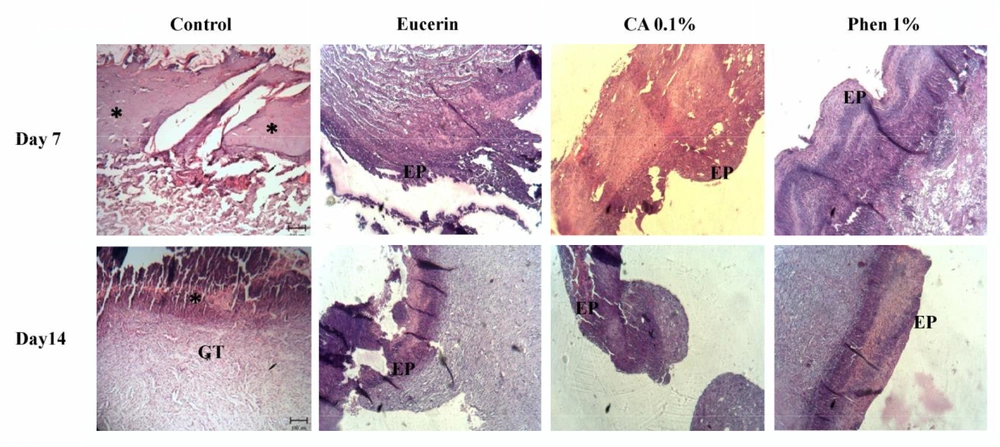
The histopathological study of the wound tissue samples of different groups was performed on days 7 and 14 after wounding. The histopathological characteristics of the tissue of all experimental groups are shown in Figures 3 and 4. The sections of no-treatment and eucerin-treated groups showed the formation of a thin epithelium layer, absence of hair follicles, and reduced blood vessels and fibroblast cells. In the phenytoin-treated groups, although the complete epithelium was formed, the hair follicles were absent and neovascularization was observed in deeper layers on day 14. In CA 0.1%-treated group, the re-epithelialization and neovascularization rates were more than those of other groups. The increased number of hair follicles and fibroblast cells were observed in CA 0.1%-treated groups.
5. Discussion
Wound-healing is the normal consequence of tissue injury that results in the restoration of damaged tissue to its normal state (2, 24). Healing a wound in the minimum time possible could improve the quality of life. The wound-healing process can be accelerated by many natural and chemical agents. In this study, we examined the effects of CA on wound healing in a full-thickness wound model in the rabbit. Cinnamic acid is a secondary metabolite of plants with broad-spectrum biological activities and low toxicity. This compound possesses anti-inflammatory, antioxidant, and antimicrobial properties (7, 11, 12). With potential for the wound-healing activity, CA is a key component of many plants such as Scrophularia striata (25, 26), Cinnamon verum (27), Inula viscosa (28), and Stevia rebaudiana and may contribute to the enhancement of wound healing by these plants.
The results of the present study showed that CA at low concentrations (< 1%) could accelerate the rate of wound closure, increase the hydroxyproline content of tissue, and improve skin wounds histologically when compared to the control group. The inflammation phase is a pivotal stage of the wound-healing process. However, the elongated inflammatory phase may delay wound healing. Cinnamic acid and its derivatives possess anti-inflammatory and free radical scavenging properties (29) that may contribute to the wound-healing activity.
Tissue injuries, ulcers, and surgical wounds may be infected by bacteria existing on the skin surface or endogenous microbes. Antimicrobial activity of CA could prevent wound infection development, resulting in wound-healing acceleration (30). According to the results of the present study, CA enhances neovascularization, which is critical for normal wound-healing. The main processes in neovascularization include the release of growth factors and proteolytic enzymes, activation of different types of cells, and synchronization of the interaction among angiogenesis factors, endothelial cells, and extracellular matrix proteins. Cinnamic acid may affect one or more of these processes, resulting in the enhancement of neovascularization (31).
Collagen is a key protein of the extracellular matrix during skin wound healing. Estimation of the collagen content of tissue is an important aspect of research toward skin wound-healing (32). In this study, the hydroxyproline (a major component of collagen) levels of skin tissue were determined, and the results revealed significantly higher levels of hydroxyproline in tissue samples obtained from the CA 0.1%-treated group than in the negative control and vehicle groups.
The results of this study revealed that CA at low doses (<1%) accelerates the wound-healing process. However, a higher dosage of 10% CA ointment was not beneficial, as shown by the results obtained from macroscopic observation, histopathological study, and hydroxyproline content determination. High doses of CA may suppress the fibroblast migration and proliferation and result in disorganized collagen production, which leads to delayed wound healing. Further studies are needed to find the precise mechanism of wound-healing activity of CA and determine the optimal dose of CA for the treatment of wounds.
5.1. Conclusion
The results of this study indicated that CA at low concentrations (< 1%) has potential for the stimulation of wound healing. Cinnamic acid accelerated the wound closure rate, augmented the hydroxyproline content, and improved skin wounds histologically. Our findings collectively demonstrated that CA could be used as a safe, efficient, and cost-effective wound-healing topical agent.