1. Background
The genus Colchicum from the Colchicaceae family is a perennial monocotyledon flowering plant with more than 160 species, which are mostly grown in full sunny plains of western regions of Asia, Europe, and east and South of Africa. In addition, the morphological characterizations of this genus are the bulb-like corms and underground ovaries (1, 2).
The Colchicum species, also known as “Sorenjân”, are important medicinal plants, which have been used for many diseases like osteoarthritis, gout, cancer, inflammations, jaundice, and sexual impotence in different societies. Pharmaceutical characteristics of Colchicum are mainly derived from its corns and seeds. The Colchicum species and other plants of the Colchicaceae family have important bioactive compounds, including alkaloids, especially tropolone alkaloids and isoquinoline alkaloids, phenolic compounds, tannins, flavonoids, and carbohydrates, which their amount depends on the growing cycles and seasons (1, 2).
Tropolone alkaloids, as the main important bioactive compounds isolated from Colchicum species, are used for the treatment of diseases like gout and cancers. Moreover, colchicine, demecolcine, and colchicoside are the main tropolone alkaloids of the Colchicum, which contain antitubullin activities and tubulin polymerization inhibitory effects. These compounds, with potential valuable biological activities, have high potential toxicity and a narrow therapeutic index. Moreover, the corms of some species have nutritive value due to high starch and low alkaloid contents (3).
Furthermore, these species also contain other compounds like isoquinoline alkaloids with anticholinesterase inhibitory activities, coumaric acid, ferulic acid, caffeic acid, vanillic acid, 2-hydroxybenzoic acid, apigenin, luteolin, and 4,6-dimethoxy-3,7- (4). The bioactive compounds of the Colchicum species, especially tropolone alkaloids, flavonoids, and phenolic acids, have anti-inflammatory and anti-arthritic activities. Colchicum autumnale L. is an important plant, which is used for the treatment of gout and osteoarthritis in traditional medicine. Furthermore, other species of Colchicum as well as Colchicum autumnale, which are, contained similar bioactive compounds, can be effective in osteoarthritis and inflammatory conditions (5, 6).
In addition, the relative toxicity of compounds isolated from some Colchicum species is well established in cell lines and animal models. According to the previous study of the author, the isolated compounds from four Colchicum species had a hemolytic activity lower than 5%, LC50 and LC90 higher than 450 and 1700 µg/mL, cytotoxic IC50 higher than 13 µg/mL, and LD50 higher than 6 mg/kg (7). Phytochemical, physicochemical, and biological evaluations of medicinal plants are the critical stage for validation of the quality of medicinal plants. Moreover, the findings of the precise phytochemical profiles can be useful to estimate the biological activities and therapeutic indices (8, 9).
2. Objectives
The current study aimed to evaluate the qualitative and quantitative phytochemical and physicochemical properties, as well as anti-inflammatory and anti-arthritic activities, of some species of Colchicum. In addition, we, firstly, evaluated the phytochemical and physicochemical properties, including preliminary phytochemical analysis, total tropolone alkaloid, total phenolic, total tannin, total flavonoid, and, secondly, performed HPLC analysis of the tropolone alkaloids and macroscopic and organoleptic properties, solubility, foreign matter, ash values, and heavy metals.
3. Methods
3.1. Plant Materials
The plant specimens, including Colchicum speciosum Steven and Colchicum robustum (Bunge) Stef., were collected at a height between 1500 to 2500 meters in the regions of Hezarjarib forests of Neka City (36.376092, 53.555002) and Badab Soort area of Kiasar City (36.353598, 53.833766) in Mazandaran Province, respectively, from October to April 2018 (Figure 1). Colchicum autumnale L. standard corms were purchased from Niak Pharmaceutical Company, Golestan Province (Iran). The plant samples were identified and confirmed by Dr. Masoud Azadbakht as taxonomic, and the representative voucher and the specimens were deposited in the herbarium of the Department of Pharmacognosy in Faculty of Pharmacy of the Mazandaran University of Medical Science. Moreover, the voucher number of these plants was defined as C. speciosum (E1-11311), C. robustum (E1-11313), and C. autumnale (BE1-11314) (10). Colchicum species and their corms are shown in Figure 2. The corms were dried at room temperature. Finally, the corms of each of the three Colchicum species were prepared for future analyses.
3.2. Preparation of Corm Extracts
All corms of the Colchicum species were separately extracted using methanol/water (80/20) solvents with the percolation method. Then, the extracts were concentrated using rota-vapor and dried using a freeze-dryer. Finally, the extracts were stored at 4°C until analysis (11).
3.3. Preliminary Phytochemical Analysis
The extract solutions of the Colchicum species (5 mL, 1 mg/mL) were used for preliminary analysis. Phytochemicals were detected using the following methods. For alkaloids, extracts were first mixed with 5 drops of HCl (1%) in the test tube and then heated for 10 min, separately. After cooling, 5 drops of Wagner reagent were dropped into the mixture. Finally, the observation of a reddish-brown precipitate indicated the presence of alkaloids. For phenolic compounds, initially, extracts were mixed with 5 drops of FeCl3 solution (0.1%) and then shacked, separately. Eventually, the observation of green or blue color indicated the presence of phenolic compounds. For tannins, extracts were mixed with 5 drops of FeCl3 solution (0.1%), separately. Finally, the observation of brownish-green or bluish-black color indicated the presence of tannins. For flavonoids, extracts were mixed with 5 drops of HCl (1%), separately. Then, a few amounts of magnesium powder were added to the mixture. The observation of red or pink color indicated the presence of flavonoids. For anthraquinones, extracts were mixed with 5 mL of HCl (1%) and 5 mL of benzene, separately. Then, 2 mL of NH4OH solution (10%) was added to the mixture and then was shacked. Observation of pink, violet, or red color in the NH4OH phase indicated the presence of free hydroxy anthraquinones. For coumarins, extracts were mixed with 5 mL of NaOH solution (10%), separately. The observation of a fluorescence bluish-green color indicated the presence of coumarins. For saponins, first, extracts were mixed with 5 mL of distilled water, separately. Afterward, it was vigorously shacked for 5 min. The formation of stable foam indicated the presence of saponins. For terpenoids and steroids, extracts were mixed with 5 mL of chloroform, separately. Then, 5 mL of concentrated H2SO4 was added to the mixture and was shacked. The observation of a ring layer with a reddish-brown color indicated the presence of terpenoids. Moreover, the red color of the mixture of extracts and concentrated H2SO4 indicated the presence of steroids. For glycosides, extracts were mixed with 5 mL of acetic acid (50%) and 5 mL of H2SO4 (3%). The observation of red color indicates the presence of glycosides. For cardiac glycosides, extracts were mixed with 5 mL of glacial acetic acid and 1 mL of FeCl3 (0.1%) solution. Then, 2 mL of concentrated H2SO4 was added to the mixture. The observation of a brown ring in the interface indicated the presence of cardiac glycosides (4, 12).
3.4. Total Analysis of Phytoconstituents
These plant species usually have many compounds, especially tropolone alkaloids, phenolic and tannin compounds, and flavonoids. For this reason, these compounds were evaluated as the main phytoconstituents (4, 13).
Total tropolone alkaloids of the corm extract of the Colchicum species were determined using the acidic potassium dichromate with UV-Spectrophotometry method, separately. Briefly, 1 g of corm extracts were dispersed in the mixture of methanol/H2SO4 solution (3%, pH = 1) with a 50/50 ratio. Then, they were extracted using sonication for 1 hour. Afterward, the methanol was evaporated by rota-vapor. Then, the final acidic extracts were decanted with chloroform, and the aqueous phase was collected and alkalized using NH4OH solution (10%, pH = 12). The solution was extracted three times with chloroform and was dried. Then, 1 mL solution of extracts (1 mg/mL) and (2.5, 5, 10, 20, and 40 mg/mL) concentrates of standard colchicine were mixed with 1 mL acidic potassium dichromate solution (pH = 2) and incubated at room temperature for 60 minutes. Finally, UV absorbances of them were measured at 352 nm, and the graph of the calibration was drawn. Moreover, these extracts were used in HPLC for the determination of any tropolone alkaloids (14, 15).
Total phenolic and tannin contents of the corm extract of the Colchicum species were determined using the Folin-Ciocalteu method separately. Briefly, 1mL of the extracts (1 mg/mL) was mixed with 1.5 mL Folin-Ciocalteu reagent and incubated at room temperature for 10 minutes. Then, 1.5 mL sodium carbonate solution (20%) was added to the mixture and incubated at room temperature for an additional 60 minutes, and absorption rates were recorded at 725 nm. A calibration curve was created using a standard concentration of gallic acid, and the total phenolic compounds of extract were determined by the calibration curve. Moreover, 100 mg polyvinyl polypyrrolidon (PVPP), 1 mL distilled water, and 1 mL of the extracts (1 mg/mL) were added into the tube and then mixed and kept at 4°C for 15 min. Afterward, it was centrifuged (3000 g for 15 min), and the supernatant was collected. The supernatant only contained simple phenolic compounds other than tannins. The phenolic content of the supernatant was measured as mentioned above. Finally, the total tannin was obtained by differences of the two above amounts (9, 16).
The total flavonoid content of the corm extract of the Colchicum species was determined using the AlCl3 method. Briefly, 1 mL of the extracts (1 mg/mL) was mixed with 1 mL of AlCl3solution (2%) and 1 mL of potassium acetate solution (10%) and incubated at room temperature for 30 min. Finally, the absorbance of the mixture was measured at 415 nm, and the total flavonoid content was determined using a standard curve with quercetin (2.5, 5, 10, 20, and 40 mg/mL) (8, 16).
3.5. HPLC Analysis of Tropolone Alkaloids
The HPLC analysis of the corm extract of the Colchicum species was done by HPLC coupled with UV spectrophotometry. The separation, detection, and assay of tropolone alkaloids (colchicine, demecolcine, 2-demethyl colchicine, 3-demethyl colchicine, colchicoside, colchifoline, cornigerine, and N-deacetyl-N-formyl colchicine isolated in a previous study of the author (7)) were performed by a HPLC Smartline Manager 5000 (Knauer, Germany) with Smartline pump 1000 and EC Nucleodur C18 column (4.6 mm × 250 mm, 5 µm particle size) at 25°C with UV detector 2500 basic model in 245 nm. Data acquisition and integration were performed with EZchrom Elite 3.2.0 software. Moreover, the confidence of the accuracy of the individuality of the peaks was obtained by standard addition and spiking method using the injection of the concentration of standard colchicine. Briefly, the extracts obtained from the abovementioned method (1 mg/mL) and the HPLC grade solutions of the compounds (125, 250, 500, 1000, and 2000 ppm) were injected into the HPLC with 20 µl volume, and the flow rate of the mobile phase was maintained at 1 mL/min. The mobile phases were HPLC grade acetonitrile (ACN) and water. Moreover, the gradient condition was as follows: 0 - 5 min, ACN 10%; 5 - 20 min, ACN 100%; 20 - 25 min, ACN 10%; 25 - 30 min, ACN 10%. Finally, the calibration curves of all compounds were obtained by plotting the peak region against the concentration of each sample, and the amount of the compounds was calculated by the obtained formulas. Moreover, it worth noting that R2 > 0.9 represents the linear measurements (2, 17, 18).
3.6. Physicochemical Properties
All physicochemical properties of the Colchicum species, including macroscopic and organoleptic properties, solubility, foreign matter, ash values, and heavy metals, were separately evaluated (19-21). Various organoleptic and macroscopic characteristics of Colchicum corms, such as powdered form color, odor, taste, and shape, were evaluated. Moreover, different reagents and solvents, including water, potassium hydroxide (10%), sulphuric acid (5%), methanol, ethanol (96%), chloroform, and hexane, were used in the solubility test. The foreign matter of the plants was determined using evaluation and weighting of non-corm components of corms. Then, one gram of powdered corms was weighted and transferred to a petri dish. Afterward, it was oven-dried at 100°C - 110°C for 1 hour. The difference in corm weight before and after warming shows the moisture content in all corm samples. Moreover, 1 gram of each powdered corms, in a weight-determined crucible, was completely incinerated in an electronic furnace for 5 - 6 hours at a continuous temperature of 500°C - 600°C. The weight of the final ash showed the total ash of all Colchicum species. According to the standard method, 10 mL of HCl (35%) and 15 mL of deionized water were added to the crucible containing the total ash of each corm sample separately and then boiled for 15 minutes. Afterward, final mixtures were filtered using ash-less filter paper and washed with hot water until they became neutral. Finally, residual ashes were dried and weighted as acid-insoluble ash. Sulphated ash was determined using 1 g of each powdered corms and 2 mL of concentrated H2SO4, by the weight-determined crucible and incineration in an electronic furnace for 30 min at 600°C. The weight of the final ash showed the sulphated ash of all Colchicum species. For the determination of water-soluble and insoluble ash in each of Colchicum corms, 25 mL of deionized water was added to the crucible containing 1 gram of total ash and was boiled for 10 minutes. Then, insoluble residues were collected on ash-less filter paper and were incinerated in an electronic furnace for 6 hours at 600°C. The final weight of ash was water-insoluble and its comparison with total ash was water-soluble ash.
The heavy metals Hg, Cu, Cd, and Pb have been determined by using the acid digestion method followed by atomic absorption spectroscopy. According to methods, 1 gram of powdered corms of all species was weighted and transferred into silica crucible separately. Then, 10 mL of HCl (70%), 5 mL of concentrated H2SO4, and 30 mL of nitric acid (70%) were added to the crucibles and were heated to boiling point for 1 hour. Finally, the solutions were filtered and were analyzed by the atomic absorption spectroscopy method. The standard curves were created using standard solutions of water-soluble salts of Hg, Cu, Cd, and Pb (HgSO4, CuSO4, CdSO4, and PbCl2 with 10, 25, 50, 100, and 500 ppm) (22).
3.7. In Vitro Anti-inflammatory Activity
According to the literature, the ability of the compounds in the prevention of protein denaturation can induce anti-inflammatory activities, especially in osteoarthritis and rheumatoid arthritis (23).
In this study, the anti-denaturation activities of the extract of the Colchicum species were evaluated separately. Briefly, 5 mL of the extract was mixed with 1 mL of Bovine serum albumin (2 % aqueous solution) and 1 mL of distilled water. Then, the mixture was incubated for 1 hour at 37°C. Afterward, the mixture was heated at 60°C for 10 min and cooled. Moreover, 3 mL of phosphate buffer saline (pH = 7.4) was added to the final mixture and was evaluated by UV spectrophotometric method at 600 nm. The anti-denaturation activities were calculated using the following formula:
Percentage of anti-denaturation activities = 100 × (Absorbance of control - Absorbance of the treated sample)/ Absorbance of control
The control solution contained the distilled water, rather than the extract. Moreover, the anti-inflammatory drug diclofenac sodium was used as the standard.
3.8. Statistical Analysis
All analytical procedures were done in triplicate and data were analyzed by SPSS and Excel. The findings are reported as mean ± standard deviation (SD).
4. Results
Table 1 shows the qualitative data of the constituents of the Colchicum species. According to the same biochemical pathways and produced compounds in all Colchicum species, similar compounds were detected in the corms of the Colchicum species. The preliminary phytochemical studies indicated the presence of alkaloids, phenolic compounds, tannins, flavonoids, coumarins, saponins, terpenoids, steroids, and glycosides. It should be noted that anthraquinones and cardiac glycosides were not detected.
| Compounds | Colchicum Species | ||
|---|---|---|---|
| C. autumnale | C. speciosum | C. robustum | |
| Alkaloids | + | + | + |
| Phenolic compounds | + | + | + |
| Tannins | + | + | + |
| Flavonoids | + | + | + |
| Anthraquinones | - | - | - |
| Coumarins | + | + | + |
| Saponins | + | + | + |
| Terpenoids | + | + | + |
| Steroids | + | + | + |
| Glycosides | + | + | + |
| Cardiac glycosides | - | - | - |
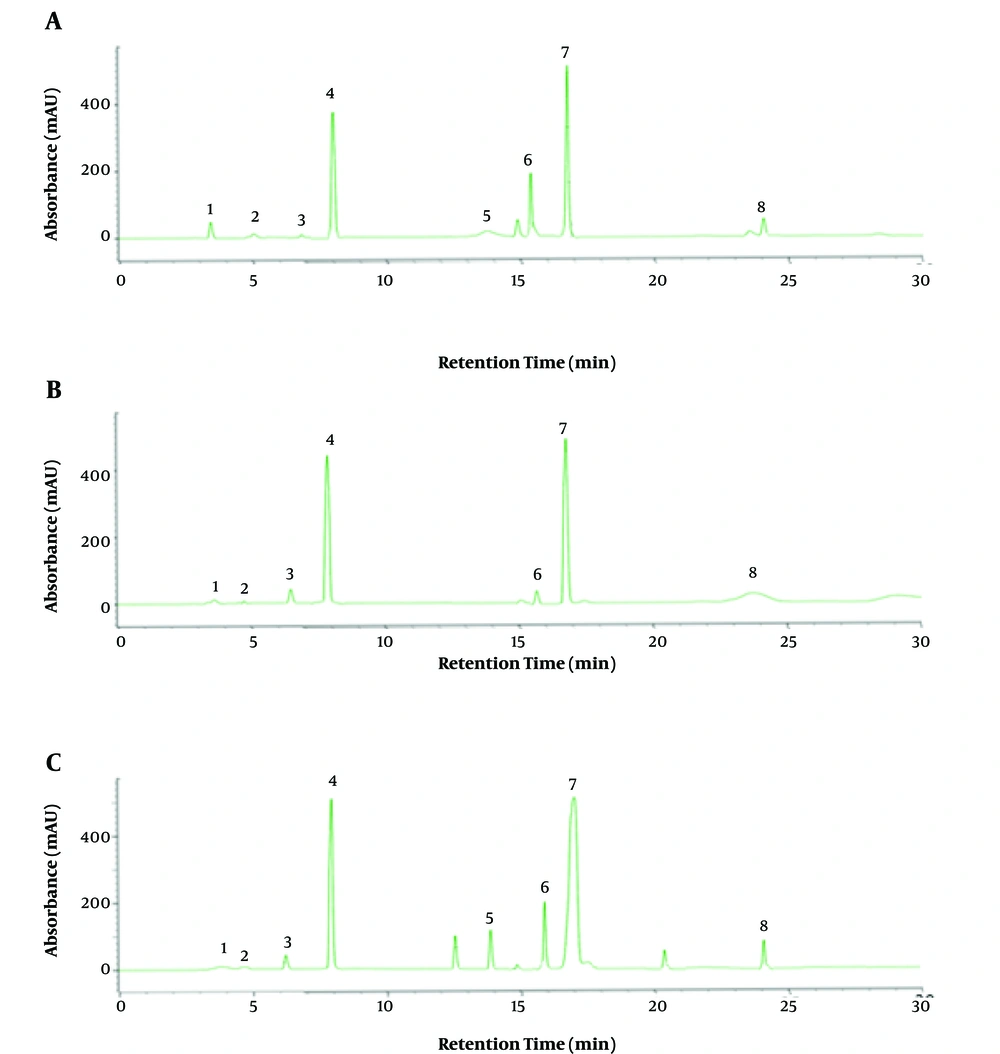
The results of the total assay of the main bioactive phytoconstituents in the corm extract of the Colchicum species are shown in Table 2. According to the results, the tropolone alkaloids were calculated in the C. autumnale and C. kurdicum with high and low amounts, respectively. Moreover, the amounts of the phenolic compounds, tannins, and flavonoids in C. autumnale, C. robustum, and C. speciosum were estimated to be higher than other species. Figure 3 and Table 3 show the chromatograms and HPLC results of the Colchicum species, respectively. The amounts of the compounds were determined by HPLC. According to the chromatograms, colchicine and demecolcine were the main compounds. Moreover, colchicine, demecolcine, and 2-demethyl colchicine were calculated in C. autumnale, C. autumnale, C. robustum with the highest amounts.
| Compounds | Colchicum Species | ||
|---|---|---|---|
| C. autumnale | C. speciosum | C. robustum | |
| Total Tropolone Alkaloid (g colchicine/100 g corm) | 0.98 ± 0.03 | 0.72 ± 0.04 | 0.64 ± 0.05 |
| Total phenol contents (g gallic acid/100 g corm) | 0.56 ± 0.04 | 0.46 ± 0.08 | 0.40 ± 0.03 |
| Total tannin contents (g gallic acid/100 g corm) | 0.07± 0.01 | 0.04 ± 0.01 | 0.10± 0.01 |
| Total flavonoid contents (g quercetin/100 g corm) | 0.37 ± 0.04 | 0.38 ± 0.02 | 0.30 ± 0.09 |
| Compounds | Colchicum Species | ||
|---|---|---|---|
| C. autumnale | C. speciosum | C. robustum | |
| Colchicoside | 15.75 ± 0.67 | 46.35 ± 1.02 | 21.92 ± 0.65 |
| 2-demethyl colchicine | 7.16 ± 0.47 | 13.18 ± 0.24 | 6.69 ± 0.18 |
| 3-demethyl colchicine | 33.23 ± 1.23 | 13.25 ± 0.34 | 36.61 ± 0.76 |
| Demecolcine | 261.51 ± 1.45 | 164.13 ± 0.43 | 192.72 ± 1.24 |
| Colchifoline | 46.26 ± 1.21 | 52.38 ± 0.15 | - |
| N-deacetyl-N-formyl colchicine | 63.69 ± 0.58 | 60.91 ± 1.45 | 34.05 ± 0.62 |
| Colchicine | 445.92 ± 1.09 | 216.56 ± 0.49 | 225.78 ± 1.31 |
| Cornigerine | 39.27 ± 0.29 | 50.06 ± 0.39 | 72.51 ± 0.75 |
The HPLC curves of the tropolone alkaloid-rich extract of the Colchicum species (A, C. speciosum; B, C. robustum; C, C. autumnale; 1, Colchicoside; 2, 2-Demethyl colchicine; 3, 3-Demethyl colchicine; 4, Demecolcine; 5, Colchifoline; 6, N-deacetyl-N-formyl colchicine; 7, Colchicine; 8, Cornigerine)
Table 4 shows the physicochemical properties of the corm of the Colchicum species. According to the obtained data, the shape of the corms varied from globular, oblong to oval. The color of the powdered corms was between grey, white, and brown. In addition, their odor and taste were spicy and bitter, respectively. Moreover, the solubility of dry powder of Colchicum species corms was estimated between sparingly soluble and freely soluble. Generally, dry powder of the corms was dissolved sparingly in water, KOH (10%), methanol, and freely in H2SO4 (5%), ethanol (96%), chloroform, and hexane. Approximately, the solubility of powdered corms of three Colchicum species in the solvents was similar to Colchicum autumnale corms.
| Characteristics | Colchicum Species | ||
|---|---|---|---|
| C. autumnale | C. speciosum | C. robustum | |
| Organoleptic properties | |||
| Shape | Globular to oval | Oblong-oval to Flat-Oval | Oblong-oval |
| Powder colour | Gray to brown | White to gray | Black gray |
| Odour | Mild spicy | Mild spicy | Mild spicy |
| Taste | Bitter | Bitter | Bitter |
| Solubility | |||
| Water | Sparingly soluble | Soluble | Sparingly soluble |
| KOH 10% | Sparingly soluble | Sparingly soluble | Sparingly soluble |
| H2SO4 5% | Freely soluble | Freely soluble | Freely soluble |
| Methanol | Sparingly soluble | Sparingly soluble | Sparingly soluble |
| Ethanol 96% | Freely soluble | Freely soluble | Freely soluble |
| Chloroform | Freely soluble | Soluble | Freely soluble |
| Hexane | Freely soluble | Freely soluble | Freely soluble |
| Foreign matter, % | 1.4 ± 0.5 | 1.5 ± 0.6 | |
| Moisture content, % | 6.5 ± 1.8 | 12.8 ± 1.4 | |
| Ash content, % | |||
| Total ash | 8.3 ± 1.1 | 8.2 ± 1.2 | 8.5 ± 1.9 |
| Acid insoluble ash | 4.5 ± 0.9 | 3.8 ± 0.9 | 4.5 ± 1.6 |
| Sulphated ash | 21.3 ± 0.3 | 28.3 ± 0.6 | 13.8 ± 0.7 |
| Water insoluble ash | 1.2 ± 0.7 | 0.87 ± 0.09 | 1.1 ± 0.3 |
| Water soluble ash | 2.8 ± 0.8 | 3.1 ± 0.58 | 2.9 ± 0.4 |
| Heavy metals, µg/kg | |||
| Hg | 0.8 ± 0.04 | 0.6 ± 0.05 | 0.3 ± 0.1 |
| Cu | 1.0 ± 0.6 | 0.8 ± 0.04 | 0.7 ± 0.1 |
| Cd | 0.07 ± 0.01 | 0.08 ± 0.001 | 0.09 ± 0.002 |
| Pb | 7.0 ± 0.1 | 0.9 ± 0.08 | 1.0 ± 0.2 |
Foreign matter percent and moisture contents of the Colchicum corms were calculated as 1.4 ± 0.54% in C. speciosum, 2.5 ± 0.67% in C. autumnale, and 12.8 ± 1.43% for C. robustum, respectively. According to the data, a foreign matter of studied samples was less than Colchicum autumnale corms. Moreover, C. robustum has higher moisture. According to obtained data, three studied species, in some cases, have higher amounts of ash values than C. autumnale. The amount of Pb is higher than the standard level in C. autumnale and C. robustum. The results of investigating anti-denaturation activities of the Colchicum species are shown in Table 5. Based on the results, C. autumnale and C. robustum had the highest and lowest anti-inflammatory activities, respectively.
| Compounds | Colchicum Species | Diclofenac Sodium | ||
|---|---|---|---|---|
| C. autumnale | C. speciosum | C. robustum | ||
| Percentage of anti-denaturation activities | 87.3 ± 1.8 | 86.2 ± 1.9 | 82.6 ± 1.6 | 94.4 ± 1.5 |
5. Discussion
In this study, phytochemical and physicochemical properties, as well as anti-inflammatory and anti-arthritic activities, of the corms of two species of Colchicum were evaluated and compared with standard corms of C. autumnale as an important medicinal plant. The preliminary phytochemical studies are useful for estimating the constituents of the plants and potential biological activities. Several studies performed a preliminary evaluation of bioactive compounds in the plants. For instance, Khan et al. (4) evaluated the phytochemical properties of Colchicum luteum. Baker reported the alkaloids, phenolic compounds, flavonoids, sterols, tannins, and saponins in the methanolic extracts of the plant corms.
Several studies have investigated the phytoconstituents. Pirildar et al. (24) evaluated the chemical constituents of different parts of the Colchicum baytopiorum CD Brickell. In this study, total alkaloids were determined using the UV spectrophotometric method. According to the results, total alkaloids were estimated at 5.27%, 2.96%, and 1.96% in the perigon, perigon tube, and leaves, respectively. Moreover, Suica-Bunghez et al. (25) evaluated the total phenolic compounds, total tannins, and total flavonoids of the Colchicum autumnale. The estimated amounts of total phenolic compounds, tannins, and flavonoids were 138.7, 82, and 66.3 mg/L, respectively.
Chan et al. (26) evaluated the total alkaloids of the cortex phellodendri using the IR method. In this study, the berberine-like compounds were also determined. Sangster and Stuart used the UV spectrophotometric methods in order to assay the alkaloids based on the backbone structures. They reported that the combination of the alkaloids with some metal salts could induce the individuality of absorption in the UV (27). Ebrahimzadeh et al. (28) evaluated the total phenolic compounds and flavonoids of some medicinal plants. In this study, the Folin-Ciocalteu and aluminium chloride methods were applied. In addition, tropolone alkaloid profiles of the Colchicum species were reported in the present study. Moreover, we studied the C. robustum for the first time. These compounds, with potent antitubullin activities, have been used in many diseases, especially gout, arthritis, and cancer. Many studies have been performed for the determination and assay of these compounds.
Ondra et al. (17) reported the amounts of colchicinoids compounds in seven Colchicum species, including C. macrophyllum, C. turcicum, C. cillicicum, C. kotschyi, C. bornmuelleri, and C. triphyllum using the HPLC method. According to this study, C. macrophyllum had higher amounts of 3-demethyl colchicine. Alali et al. (2) evaluated the colchicine content of Colchicum brachyphyllum and Colchicum tunicatum by the HPLC method. In this study, the estimated amount of colchicine in all species ranged from 0.02% to 0.15%. Al-Fayyad et al. (3) calculated the amount of colchicine in the C. Hierosolymitanum under cultivation, which was estimated at 0.766 mg/g of the dry weight of the corms. Physicochemical properties, especially organoleptic and macroscopic properties, solubility in different solvents, amounts of foreign matter, moisture contents, all ash values, and amount of heavy metals of medicinal plants, are important factors that have been used for the determination and validation of quality controls (29, 30).
Some studies explained the applications of physicochemical properties in quality control assessments of medicinal plants. Moreover, in all herbal medicine pharmacopeias, especially British, German, and Iranian herbal pharmacopeia, physicochemical analysis is the primary important test for validation of approved medicinal plants (31). In the present study, shape, powder color, odor, and taste of corms, solubility in the several solvents, percentage of foreign matter, moisture contents, ashes, and the amount of heavy metals of the Colchicum species were evaluated, separately. Moreover, the physicochemical properties of the corms of two endemic species, C. speciosum and C. robustum were compared with C. autumnale standard corm. Notably, the physicochemical properties of other medicinal plants such as Colchicum luteum, Piper callosum, Salacia macrophylla, and Heracleum persicum are evaluated by some previously performed studies (32-35).
According to the findings of physicochemical analysis of Colchicum luteum, organoleptic of the corm was determined as ovoid shape and bitter taste. In addition, moisture content of C. luteum was calculated less than 6%, and total ash, acid insoluble ash, water-soluble ash, and water-insoluble ash were 1.33%, 6%, 1.24%, and 4.28%, respectively, whereas, moisture contents of C. autumnale, C. speciosum, and C. robustum were calculated as 8.7 ± 1.05%, 5.4 ± 1.53%, 6.5 ± 1.84%, and 12.8 ± 1.43%, respectively. In another study, physicochemical properties of Heracleum persicum, including ash values and moisture content, have been evaluated. In all plant organs, the total ash was determined at 2% - 10% and the moisture contents were 6% - 10%.
Furthermore, the morphoanatomical and physicochemical profiles of the Piper callosum, as the quality control parameters, were evaluated. In the present study, moisture content, total ash, sulphated ash, and acid insoluble ash were calculated as 8.6%, 11.25%, 68.02%, and 2.82% of the leaf and 6.1%, 5.25%, 12.5%, and 0.27% of the stem, respectively. In addition, the amount of Cd, Cu, and Hg were estimated as 0.03 mg/kg, 9.5 mg/kg, and 0.02 mg/kg in the leaf, respectively. Higher amounts of moisture were associated with induced risk of various microbial and insect contaminations. In the present study, C. robustum has a higher amount of moisture and an enhanced risk of various contaminations. Different ash values, which are used for the determination of the total properties of the compounds of medicinal plant, have been evaluated in the powdered corms of Colchicum species. The amount of heavy metals in medicinal plants is an important parameter, which can be used to perform quality control. Heavy metals such as Hg, Cu, Cd, and Pb have the standard amounts in plants and foods. Moreover, in high amounts, they can induce several complications such as cerebral and cardiovascular disorders. According to standardization references, the maximum amount of Hg, Cu, Cd, and Pb were estimated at 4, 6, 0.1, and 0.6 µg/kg, respectively (36, 37).
Some studies illustrated the anti-inflammatory effects of medicinal plants. Williams et al. (38) reported a novel method for the rapid determination of anti-inflammatory activities of the compounds. Moreover, they suggested that the anti-denaturation compounds are effective for improving inflammatory conditions such as rheumatoid arthritis and osteoarthritis. Rahman et al. (23) evaluated the in vitro anti-arthritic activities of the Oryzae sativa var. Joha Rice. According to this study, the anti-inflammatory and anti-arthritic activities with anti-denaturation effects of the plant were 40% to 60%. Ramalingam et al. (39) reported that the anti-denaturation effects of the Ziziphus oenoplia ranged from 50% to 98% for aqueous, ethanolic, and ethyl acetate extracts. According to this study, other studied species of Colchicum, as well as C. autumnale, has the same bioactive compounds and are represented as valuable medicinal plants.
5.1. Conclusions
According to the findings of the present study, the corm of the Colchicum species contained the same main compounds with different amounts, as well as appropriate physicochemical properties. These plants with high efficacies in inflammatory diseases can be used for the treatment of arthritic diseases. In addition, the phytochemical profiles, physicochemical properties, and biological activities were evaluated. These findings can help to ease the difficulty of the standardization and formulation of the Colchicum-based drugs. Moreover, the valuable biological effects of these plants can stimulate large-scale cultivations.