1. Background
The testes are male gonads responsible for the production of sperm and secretion of the male sex hormone, testosterone. The testes are two oval-shaped organs located in the scrotum behind the penis (1). Impairment in the reproductive function of the testis could impede its major roles in spermatogenesis and production of androgens, subsequently leading to infertility. One of the common factors responsible for testicular dysfunction is an elevated level of free radicals in the male gonads (2). Exposure to heavy metals, even in a minute quantity, could generate free radicals in men. Common examples of heavy metals include cadmium, mercury, lead, chromium, and copper.
Cadmium is well known to be a prime threat to the environment (3). It is naturally occurring in the earth’s crust and is primarily found as an impurity in zinc and lead deposits. In industries, it is used to make television screens, paint pigments, lasers, cosmetics, batteries, and galvanizing steel and is found mostly in tobacco cigarettes. Living organisms, including humans, are exposed to cadmium mainly by ingestion and inhalation. It has been revealed that exposure to even a low level of cadmium (1 - 2 mg per kilogram body weight) adversely affects the kidneys, lungs, bones, and testes (4). The main mechanism by which cadmium exerts its toxicity effect is by increasing oxidative stress through the overproduction of free radicals, leading to elevated lipid peroxidation, depleted sulfhydryl groups, and reduced glutathione. This is extremely hazardous to cells and could alter proteins, lipids, and enzymes and even induce DNA damage (5).
Reports suggest that cadmium toxicity can be ameliorated by the use of antioxidants and medicinal plants such as beetroot juice, which have a protective role in the male reproductive system of rats against cadmium chloride toxicity (6). The ameliorative effect of Allium cepa on testicular toxicity following cadmium exposure in rats has been reported (7). Other plants possessing good antioxidant potential and reproductive-enhancing properties could also be used to ameliorate the effect of cadmium toxicity in rats; one of such plants is Agnus castus leaves.
Vitex Agnus castus (family: Lamiaceae) is commonly called vitex, chaste tree, or monk’s pepper. It is known among Yorubas as Ata-iyere (8). Flavonoids, essential oils, lignans, diterpenoids, and glycosides constitute major classes of phytoconstituents of the genus vitex (9). Agnus castus has been used in the treatment of many female conditions, including premenstrual dysphoric disorder (PMDD), premenstrual syndrome (PMS), infertility, disrupted lactation, hyperprolactinemia, corpus luteum insufficiency, and acne (9). Its lethal dose is 17.21 g/kg body weight (10). Agnus castus is among the most dominant plants containing a high level of flavonoids, which help in protecting cells from harm by regulating cell oxidative damage, reducing organ damage, and improving the balance between oxidants and antioxidants (8, 11).
2. Objectives
The present study aimed at investigating the effect of Agnus castus aqueous extracts on testicular function indices in cadmium chloride-administered rats.
3. Methods
3.1. Plant Material
Agnus castus leaves were authenticated at the herbarium of the Plant Biology Department, University of Ilorin, Nigeria. A voucher sample number UILH001/1345 was deposited at the herbarium. The leaves were dried, pulverized, and extracted in distilled water (solvent). The filtrate was concentrated using a water bath, and subsequent analysis was carried out by reconstituting the concentrated filtrate in distilled water.
3.2. Experimental Animals
Forty healthy, adult male rats (100 - 160) g were obtained from the Animal Holding Unit of the Department of Biochemistry, University of Ilorin, Ilorin, Kwara State. The animals were housed in well-ventilated rooms (photo-period; 12-h light and 12-h dark cycle; temperature 25°C - 27°C) and fed with rat chow and water. They were allowed to acclimatize for two weeks before the beginning of the administration.
The rats were randomly grouped into five groups, each containing five animals. The animals were treated as follows: Group A (control group) received distilled water, group B received 6.5 mg/kg body weight of cadmium chloride, group C received 6.5 mg/kg body weight of cadmium chloride with 50 mg/kg of aqueous leaf extract of Agnus castus, group D received 6.5 mg/kg body weight of cadmium chloride with 100 mg/kg of aqueous leaf extract of Agnus castus, and group E received 6.5 mg/kg body weight of cadmium chloride with 200 mg/kg of aqueous leaf extract of Agnus castus. The administration was carried out by using an oropharyngeal cannula, once daily for 21 days. The animals were sacrificed on day 22. The testis was excised and weighed and then immersed in an ice-cold 0.25 M sucrose solution. The testes were then homogenized, and the resulting supernatant was stored frozen for biochemical assays.
3.3. Assay Kits
An assay kit for cholesterol using the CHOD-PAP reaction at 546 nm was used to determine its concentration. This was a product of Spectrum Diagnostics Ltd., Cairo, Egypt. Testosterone, follicle-stimulating hormone, and luteinizing hormones were the products of Monobind Inc., Lake Forest, USA. All other reagents used were of analytical grade and prepared in volumetric flasks using distilled water.
3.4. Other Biochemical Assays
Testicular function indices such as proteins were determined as described by Gornall et al. (12), sialic acid as described by Warren (13), and activities of acid phosphatase ACP and alkaline phosphatase ALP as described by Wright et al. (14, 15). Lipid peroxidation was determined based on the method described by Varshney and Kale (16). Antioxidants such as superoxide dismutase (SOD) (17), catalase, and reduced glutathione activities were determined by Beers and Sizer (17), Misra and Fridovich (18), and Jollow et al. (19), respectively.
The biuret method described by Gornall et al. (12) was used to survey the total testicular protein concentration. The assay mixture comprised 1.0 mL of the sample (testicular supernatant) and 4.0 ml of Biuret reagent. The mixture was shaken properly and allowed to stand for 30 min at room temperature. The blank was prepared by replacing the sample with distilled water. Absorbance was read against blank at 540 nm. The concentration of proteins in the sample was then estimated by extrapolating from a standard curve of bovine serum albumin (BSA, 1 - 10 mg/mL).
The testicular sialic acid concentration was determined by using the protocol of Warren (13). Equal volumes of the testicular supernatant and 0.1 M sulphuric acid were heated for 1 h at 80°C. From this heated mixture, a sample of 0.2 mL was removed into a test tube, and 0.1 mL of periodate solution was added and left to stand for 20 min. Subsequently, 1 mL of arsenite solution was added, and the test tubes were shaken until a yellow-brown color diminished. Thiobarbituric acid (3 mL) was then added into the test tubes, plugged with cotton wool, and heated vigorously in boiling water for 15 min. The test tubes were then removed and placed in cold water for 5 min. From this solution, 1 mL was taken to fresh test tubes containing 1 mL of cyclohexanone. The test tubes were then shaken vigorously. The absorbance of the upper red-colored cyclohexanone part was taken against the blank at 549 nm. The blank was replaced with distilled water instead of the sample.
The activity of acid phosphatase was measured using the spectrophotometric method described by Wright et al. (14). The assay mixture consisted of 2.2 mL of sodium acetate buffer (pH = 4.5) and 0.2 mL of appropriately diluted test sample while the blank contained distilled water instead. This was left to equilibrate for 10 min. Then, p-nitrophenyl phosphate 10 mM (0.5 mL) was added, mixed, and incubated for 30 min at 37°C. The reaction was then ended with the addition of 2 mL of 1 N sodium hydroxide. The optical density was read at 400 nm and enzymatic activity was measured using the following formula:
Where: OD/min = change in optical density of reaction mixture per mixture; TV = total volume of the reaction mixture; F = total dilution factor; SV = volume of enzyme source; L = lightpath length (1 cm); 9.9 = extinction coefficient of 1 µM of p-nitrophenol in an acid solution of 1 mL volume and 1 cm path length; 1000 = factor introduced to enable the enzymatic activity to be expressed in nM/min /mg protein.
3.4.1. Alkaline Phosphatase
Alkaline phosphatase activity was measured using the protocol described by Wright et al. (15). The assay tube contained 2.2 mL of 0.1 M carbonate buffer, 0.1 mL of 0.1 M MgSO4.7H2O, and an appropriately diluted sample. The mixture was then incubated at 37°C for 10 min. Then, 0.5 ml of 10 mM p-nitrophenyl phosphate was added to the mixture. After incubation, the mixture was again incubated at 37°C for 30 min, and the reaction was ended with 1 N sodium hydroxide. The blank was constituted by exchanging the sample with 1 mL of distilled water. The absorbance was read against the blank at 400 nm and the activity of the enzyme was determined using the same formula used for acid phosphatase.
3.4.2. Lipid Peroxidation
The spectrophotometric thiobarbituric acid reactive substances (TBARS) method described by Varshney and Kale (16) was used for the calculation of malondialdehyde formed, as an estimation of lipid peroxidation. The sample (1 mL) was centrifuged at 17,000 g for 20 min. Then, 0.4 mL of the supernatant was mixed with 1.6 mL of 0.15 M TRIS KCl buffer to which, 0.5 mL of 30% trichloroacetic acid was added. Next, 0.5 mL of 0.75% thiobarbituric acid was added and heated for 45 min at 80°C. The mixture was then cooled in ice and centrifuged at room temperature for 10 min at 3,000 rpm. The absorbance was then taken against a reference blank of distilled water at 532 nm.
3.4.3. Catalase
The activity of catalase was determined as described by Beers and Sizer (17). The assay mixture containing 1 mL of hydrogen peroxide (H2O2) and an aliquot (0.1 mL) of the enzyme source (homogenate) was incubated in the spectrophotometer for 5 min to establish the blank rate and temperature equilibration. The absorbance of the blank reference of distilled water was read at 240 nm. A decrease in absorbance was monitored at 240 nm for 2 min. A change in absorbance (ΔA240/min) was calculated using the following expression:
3.4.4. Superoxide Dismutase
The superoxide dismutase assay was done according to a method by Misra and Fridovich (18). The assay tube contained 0.2 mL of the sample, 2.5 mL of 0.1 M phosphate buffer (pH = 10.2), and 0.3 mM adrenaline. The same was repeated for the blank, but the sample was replaced with distilled water. An increase in absorbance was monitored every 30 s for 150 s at 480 nm. Percentage inhibition was calculated as follows:
3.4.5. Reduced Glutathione
The reduced glutathione level was determined as described by Jollow et al. (19). The principle works with the oxidation with sulfhydryl reagent 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) to form the yellow derivative 5-thio-2-nitrobenzoic acid (TNB) measurable at 412 nm. An aliquot of the homogenate was added to an equal volume of 4% sulfosalicylic acid to deproteinize the sample. The mixture was centrifuged at 3,000 rpm for 15 min at 2°C using a Uniscope laboratory centrifuge device. Ellman reagent (4.5 mL) with 0.5 mL of the supernatant was then added and measured at 412 nm, while the blank reference was prepared with 0.5 mL of sulfosalicylic acid in 0.1 M phosphate buffer and 4.5 mL of Ellman reagent.
3.5. Statistical Analysis
Data were expressed as the mean ± SEM of four measurements. The data were subjected to statistical analysis using Dunnett’s multiple comparisons test with a one-way analysis of variance (ANOVA). The analyses were done with Graph Pad Prism, version 6.01 (Prism 6, Graph pad from Japan). Differences were considered statistically significant at P < 0.05.
4. Results
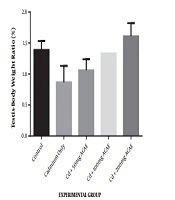
The effect of the aqueous extract of Agnus castus leaves on the testes to bodyweight ratio is displayed in Figure 1. There was an increase in the testes to body weight ratio observed as 1.06%, 1.33%, and 1.61% at the various doses of 50, 100, and 200 mg/kg body weight of aqueous leaf extract of Agnus castus, respectively, while the ratio in controls was 1.39%. This increase was only observed in rats administered with the highest dose of the extract. The increase, however, was not significant.
4.1. Testicular Function Indices
4.1.1. Testicular Cholesterol
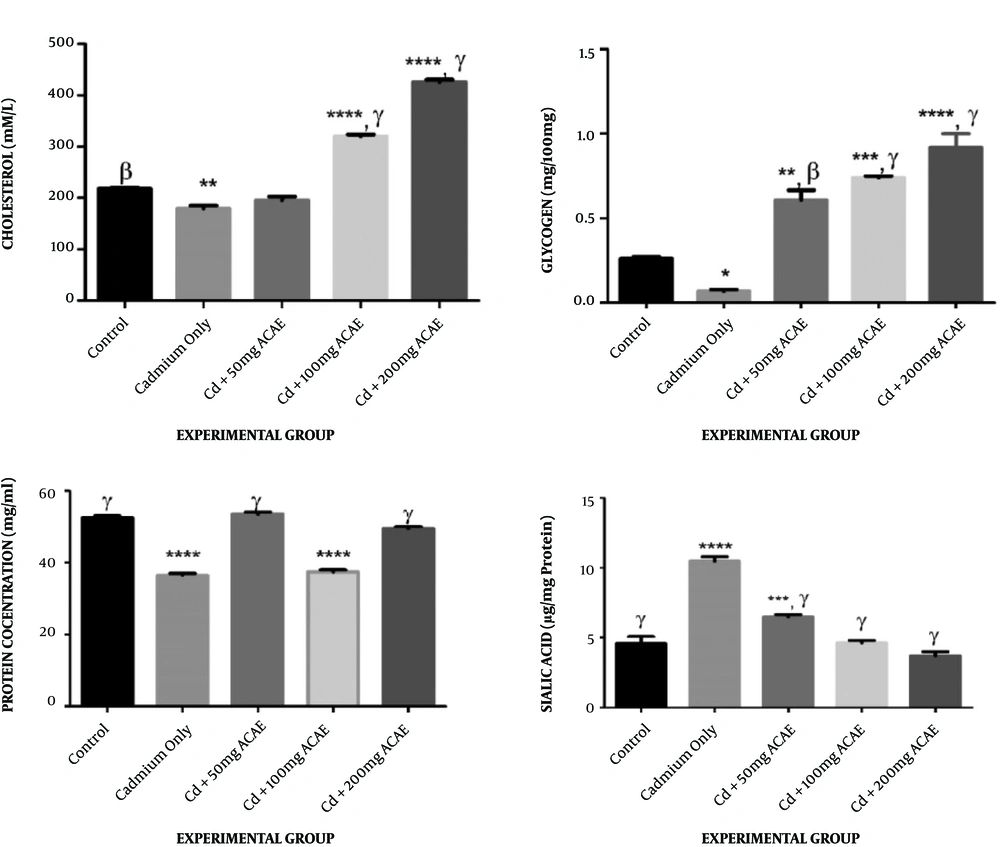
Testicular cholesterol levels after treatment with cadmium chloride and aqueous leaf extract of Agnus castus were observed to be 208.46, 336.44, and 448.16 mM at various doses of 50, 100, and 200 mg/kg body weight of the extract administered when compared to the level in the control group, which was 219.74 mM. A significant increase in the testicular cholesterol level was seen in animals administered with 100 and 200 mg/kg body weight of the extract. There was, however, a significant decrease in the testicular cholesterol level of rats that received cadmium chloride only (186.28 mM) when compared to the control group (Figure 2A).
Effect of Agnus castus aqueous leaves extract on testicular cholesterol, glycogen, protein, and sialic acid concentration. Each value represents the mean of five replicates ± SEM.*, represents statistical difference relative to controls at P < 0.05. * is significant at P < 0.05, ** at P < 0.01, *** at P < 0.001, and **** at P < 0.0001. α is significant at P < 0.05, β at P < 0.01, and γ at P < 0.001 versus cadmium only.
4.1.2. Testicular Glycogen
Treatment of rats with cadmium chloride and Agnus castus aqueous leaves extract resulted in significant increases in testicular glycogen, across all treated groups, to 0.62, 0.76, and 0.95 mg/100 mg at various doses when compared to controls that showed 0.26 mg/100 mg. Animals that received cadmium chloride only, however, showed a significant decrease in their testicular glycogen concentration, which was 0.057 mg/100 mg, when compared to the control group (Figure 2B).
4.1.3. Testicular Protein
Figure 2C displays the effects of treatment with cadmium chloride and aqueous extract of Agnus castus leaves on the testicular protein content of rats. Rats that received 50 and 200 mg/kg body weight of the extract did not display any significant difference (52.52 and 51.68 mg/mL, respectively) from the controls (51.60 mg/mL), whereas there was a significant reduction in the testicular protein level of rats that received 100 mg/kg body weight of the extract which was observed to be 37.18 mg/mL. Animals administered with cadmium chloride only also displayed a significant decrease in the testicular protein level, when compared to controls (Figure 2C).
4.1.4. Testicular Sialic Acid
The trends showing the effect of co-treatment with cadmium chloride and aqueous extract of Agnus castus leaves on testicular sialic acid are shown in Figure 2D. There was a significant increase in the sialic acid concentration in rats that received cadmium chloride only and 50 mg/kg body weight of Agnus castus aqueous leaves extract (6.59 and 10.25 mg/g, respectively), whereas there was no significant difference between rats that received 100 and 200 mg/kg body weight of the extract (4.56 and 3.75 mg/g, respectively) and controls that showed 4.43 mg/g (Figure 2D).
4.2. Antioxidant Activities
4.2.1. The Effect of Aqueous Extract of Agnus castus Leaves on Catalase, Reduced Glutathione, Superoxide Dismutase, and Malondialdehyde
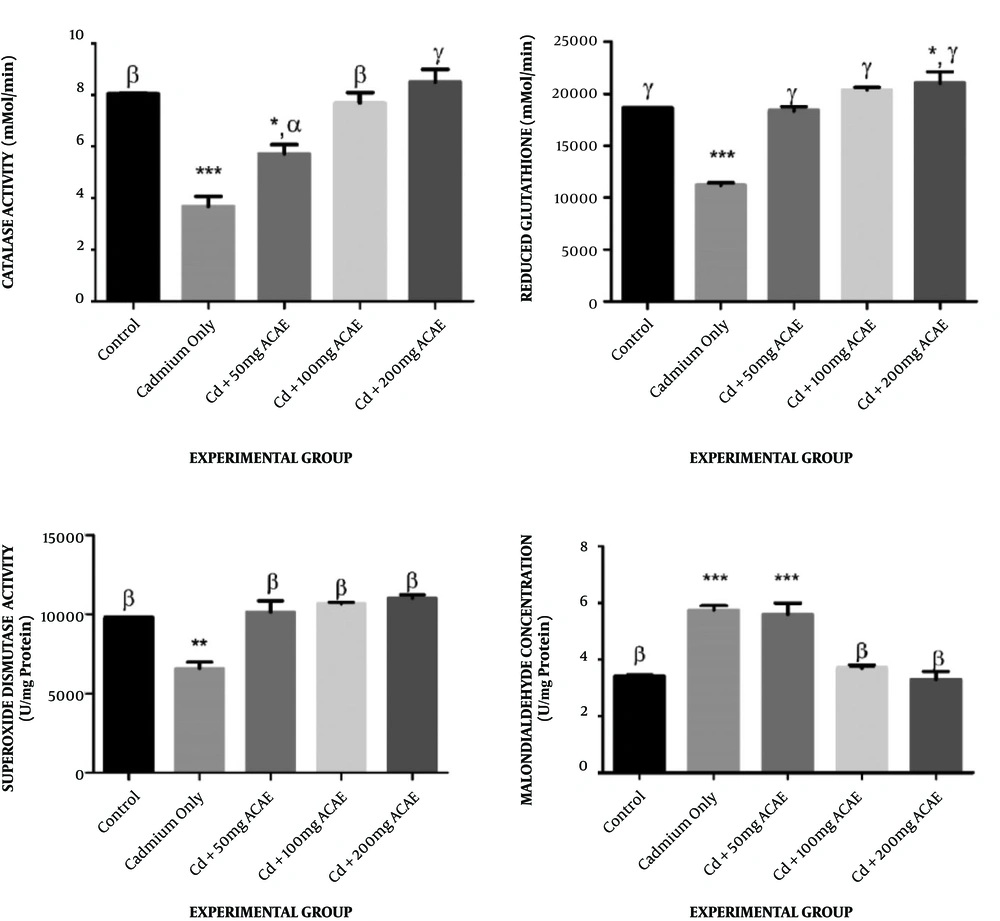
The trends in catalase activities of rats administered with cadmium chloride and Agnus castus aqueous leaves extract (Figure 3A) showed an insignificant change in animals that received 100 and 200 mg/kg body weight of extract (8.09 and 8.99 mMol/min, respectively) when compared to the control of 8.07 mMol/min, whereas there was a significant decrease in the activities of catalase in animals administered with cadmium chloride only and the lowest dose of extract, 50 mg/kg body weight (6.07 and 4.06 mMol/min, respectively).
Effect of Agnus castus aqueous leaves extract on catalase, reduced glutathione, superoxide dismutase activity, and malondialdehyde. Each value represents the mean of five replicates ± SEM. *, represents statistical difference relative to controls (distilled water) at P < 0.05. * is significant at P < 0.05, ** at P < 0.01, *** at P < 0.001 and **** at P < 0.0001. α is significant at P < 0.05, β at P < 0.01, and γ at P < 0.001 versus cadmium only
Rats that were co-treated with Agnus castus aqueous extract and cadmium chloride had their reduced glutathione levels increased to 18768.8, 20665.7, and 22115 U/mg protein, respectively, when compared to the control of 18666.7 U/mg protein, while rats that were given cadmium chloride only showed a significant decrease in the reduced glutathione level of 11429.2 U/mg protein when compared to the control (Figure 3B).
Treatment with cadmium chloride and aqueous extract of Agnus castus leaves was observed to result in an insignificant change in SOD activities, across all extract-treated groups to 9837.5, 10740.7, and 11230 U/mg protein at various doses when compared to the control of 9800 U/mg protein. There was, however, a significant decrease in SOD activities of rats that received cadmium chloride only (6983.33 U/mg protein) when compared to the control (Figure 3C).
Malondialdehyde concentrations of animals co-treated with Agnus castus aqueous extract and cadmium chloride were observed to increase significantly in rats that received cadmium chloride only and 50 mg/kg body weight of extract (5.90 and 5.52 µg/L, respectively) compared to the control of 3.45 µg/L, while there was no significant change in the malondialdehyde concentration of rats that received 100 and 200 mg/kg body weight of extract (Figure 3D).
4.3. Hormones
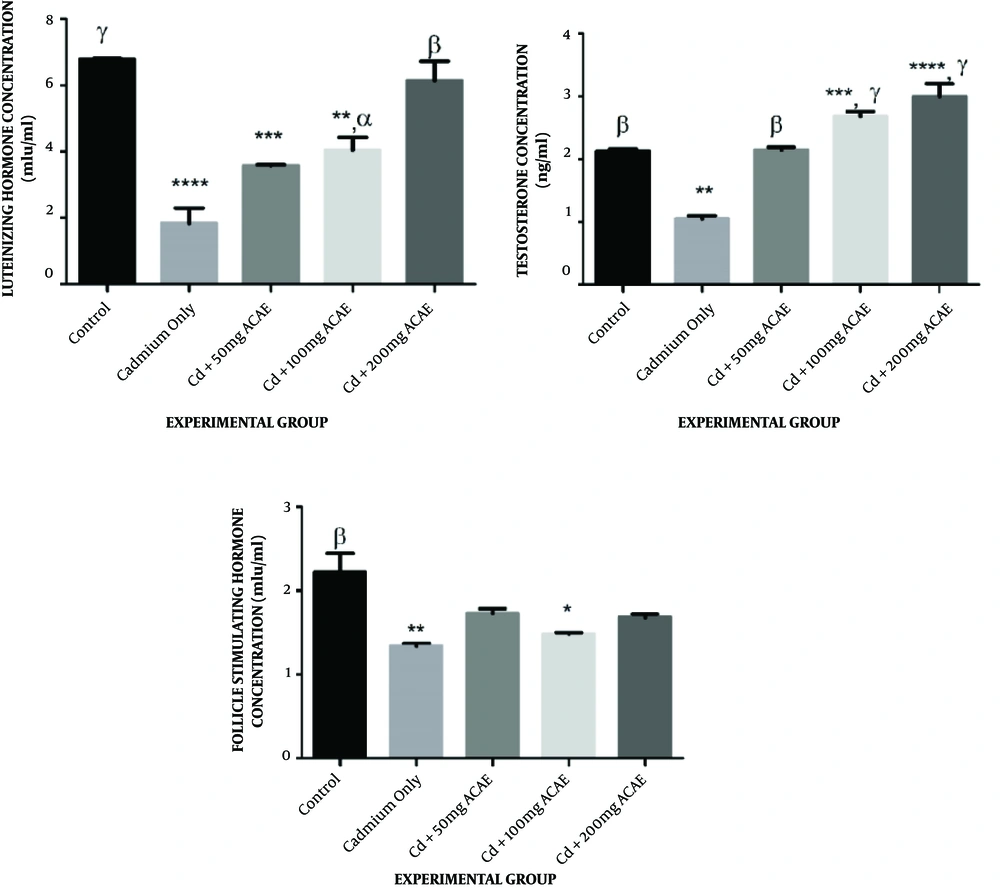
4.3.1. Effect of Aqueous Extract of Agnus castus Leaves on Serum Luteinizing Concentration
In the case of serum luteinizing hormone levels of rats that received cadmium chloride and aqueous extract of Agnus castus leaves, there was a reduction in the LH level to 3.61, 4.13, and 6.03 µg/mL at various doses administered compared to the control (6.81 µg/mL). This was significant in rats that received cadmium chloride only (1.98 µg/mL), 50, and 100 mg/kg body weight of extract (Figure 4A).
Effect of aqueous extract of Agnus castus leaves on serum testosterone, luteinizing hormone, and follicle-stimulating hormone concentration. Each value represents a mean of five replicates ± SEM.* represents statistical difference relative to controls at P < 0.05. * is significant at P < 0.05, ** at P < 0.01, *** at P < 0.001, and **** at P < 0.0001. α is significant at P < 0.05, β at P < 0.01, and γ at P < 0.001 versus cadmium only.
4.3.2. Effect of Aqueous Extract of Agnus castus Leaves on Serum Testosterone Concentration
Serum testosterone concentrations of animals that were co-treated with cadmium chloride and Agnus castus aqueous extract were observed to increase to 2.19, 2.76, and 3.20 µg/mL at various dosages administered, which was significant in rats that received 100 and 200 mg/kg body weight of extract compared to the control of 2.36 µg/mL, whereas there was a significant reduction in the serum testosterone concentration of animals that received cadmium chloride only to 1.10 µg/mL when compared to the control (Figure 4B).
4.3.3. Effect of Aqueous Extract of Agnus castus Leaves on Serum Follicle-stimulating Hormone Concentration
Concerning the follicle-stimulating hormone concentration, however, there were decreases in the level of FSH across all treated groups from the control of 2.15 µg/mL to 1.79, 1.50, and 1.72 µg/mL, across the extract-treated groups, as well as in cadmium chloride administered rats (1.07 µg/mL). This decrease was only significant in cadmium chloride administered rats and rats that received 100 mg/kg body weight of extract (Figure 4C).
5. Discussion
In the current study, exposure of rats to cadmium chloride only caused a substantial reduction in the weight of testes when compared to rats in the control group although this was not statistically significant. When cadmium chloride was co-treated with Agnus castus, there was an insignificant increase in testicular weight compared to rats maintained on cadmium chloride alone. Changes in organ weight have been used as an index of toxicity of metals since it could reveal swelling in organs, and the organ to body weight ratio helps define the general health status of experimental rats (20). The decrease observed in testicular weight in this study could be ascribed to the toxic effects of cadmium. Ekhoye (21) have also reported a similar reduction in the testes to body weight ratio after the administration of cadmium chloride to male Wistar rats.
The study revealed that exposure of rats to cadmium chloride only caused a reduction in the testosterone concentration. There were significant increases in all the groups when compared to the Cd dose. Compared to the control, Cd + 100 mg ACAE and Cd + ACAE groups were significantly increased and restored the effect of cadmium in the testis. The significant reduction in the testosterone concentration in cadmium chloride-only treated rats could be due to a decrease in the secretion of luteinizing hormone concentration, its precursor. Testosterone is the primary male sex hormone secreted by Leydig cells in the testis by the stimulation of the luteinizing hormone and functions in maintaining the structural integrity of the testis and other sex organs (22). Treatment with Agnus castus aqueous leaves extract was, however, able to elevate the concentration of this hormone, restoring it to values related to the control, and even above it. This finding agrees with a previous study where there was an increase in the testosterone concentration in male Wistar rats following the administration of the leaf extract of Cissus populnea (23).
The luteinizing hormone concentration showed a significant decrease in all groups except for the group fed with Cd + 200 mg ACAE. Compared to the Cd-only group, there was also a significant increase across the groups. The luteinizing hormone (LH) is one of the hormones secreted by the anterior pituitary gland, and the concentrations of these hormones in the blood are usually decreased by cadmium chloride (24). The decrease in its concentration in rats administered with cadmium chloride-only, as well as in rats that received the low and medium extract dosages (50 and 100 mg/kg body weight) could imply that the Agnus castus aqueous leaves extract was not able to combat the toxic effect of cadmium at the dosages administered. The highest dose of Agnus castus aqueous leaves extract (200 mg/kg body weight) was, however, able to restore the LH concentration to values comparable with the control. The finding agrees with the results of a previous study where an aqueous extract of Aspilia africana leaves reversed the effect of cadmium chloride-induced perturbations in the testes of adult Wistar rats (25). In conjunction with other sex hormones, the role of follicle-stimulating hormone (FSH) is to sustain the Sertoli cells in the testis, hence maintaining spermatogenesis (26). There was a decrease across all the groups when compared to the control. A significant decrease in the FSH concentration in cadmium chloride-only and Cd + 100 mg ACAE treated rats could suggest an impairment in spermatogenesis as a result of probable Sertoli cells’ dysfunction, whereas treatment with Agnus castus aqueous leaves extract did not seem to restore the level of FSH to normal. Cadmium is such an endocrine disruptor, altering the level of the female sex hormones, including FSH from previous studies (25, 27-29).
The testicular protein concentration across the groups showed that Cd only and Cd + 100 mg ACAE were significantly reduced compared to the control. A decrease in the testicular protein level, which may imply decreased protein biosynthesis, could lead to a reduction in sperm maturation. The protein has been reported to be a vital requirement for sperm maturation and spermatogenesis (30). The restoration of protein concentration in the testes on co-treatment with the aqueous extract of Agnus castus leaves suggests the restoration of protein alterations.
In this study, testicular glycogen was increased in all groups co-administered with Cd and ACAE when compared to the control, while there was a reduction in cadmium-treated rats. Cadmium-only-treated rats compared to other groups, also had significant increases in Cd + ACAE treated rats. Cadmium chloride-treated rats showed a significant decrease in their testicular glycogen level, which may be due to a reduction in energy production required for the proper functioning of the testes (31). A subsequent dose-dependent increase in the glycogen level after extract administration could infer that aqueous leaf extract of Agnus castus successfully ameliorated the toxic effects of cadmium chloride on the glycogen concentration and was able to restore it to a normal level. Positive testicular energy metabolism is also required to maintain spermatogenesis, as a previous study revealed that testicular cells rely on glycogen for vitality and development of male gonad (25).
The testicular sialic acid level showed significant increases in the groups fed with cadmium only and Cd + 50 mg ACAE when compared to the control, while there was a significant decrease across all other treated groups. The current study observed a significant increase in sialic acid concentration in rats that received cadmium chloride only. This might indicate an underlying problem with the testes, as overproduction of sialic acid in tissues could be an indication of disease (32). Aqueous leaf extract of Agnus castus was, however, able to reduce the concentration of sialic acid significantly to the normal control value in rats that received 100 and 200 mg/kg body weight of the extract.
The cholesterol level showed a significant decrease in the cadmium group and a significant increase in the Cd + 100 mg ACAE and Cd + 200 mg ACAE groups. Testicular cells require cholesterol for two reasons: Membrane biogenesis and cell signaling, as well as a precursor for androgen synthesis (33). The total cholesterol level significantly decreased after treatment with cadmium chloride, which may imply a resultant reduction in the synthesis of steroid hormones including testosterone since cholesterol is the precursor for steroid hormones. This was, however, ameliorated to concentrations similar to the control only in rats that received 50 mg/kg body weight of the extract.
In addition to a decrease in the cholesterol concentration observed in cadmium chloride administered rats, the lipid peroxidation level, concerning the malondialdehyde concentration, significantly increased in rats that received cadmium chloride-only but was restored by Agnus castus aqueous leaves extract to values close to the control in rats that received 100 and 200 mg/kg body weight of the extract. This seemed to validate the theory that cadmium chloride triggers the generation of reactive oxygen species and other free radicals, resulting in a depletion in the activity of antioxidant enzymes and glutathione (34-36).
There was a significant decrease in the activities of all antioxidants and antioxidant enzymes assayed for in the current study in cadmium-only-treated rats compared to the control. These activities, however, increased on co-administration with Agnus castus aqueous leaf extract at different dosages used. Decreased activity of superoxide dismutase and catalase in cadmium chloride-only treated rats could suggest that the effect of free radicals on the cells have outweighed the activities of these antioxidant enzymes, which could subsequently predispose cells to further free radicals’ attack. Superoxide dismutase and catalase are first-line defense antioxidants involved in the dismutation of superoxide anion radical, decomposing hydrogen peroxides and hydroperoxides to harmless substances (37). The glutathione concentration also followed a similar trend with other antioxidants assayed for in this current study, corroborating the possibility of free radicals’ attack on tissues as a result of cadmium intake. Glutathione is a second-line defense antioxidant, also referred to as a scavenger (37). Jahan et al. (38) also reported findings and trends similar to the current study.
5.1. Conclusions
Conclusively, in cadmium chloride only treated animals, there was a reduction in the activities of antioxidant enzymes, antioxidants, and other testicular function indices. However, Agnus castus aqueous leaf extract could effectively increase the level of reduced glutathione, catalase, superoxide dismutase, testosterone, luteinizing hormone, total protein, and glycogen in the testes of cadmium chloride administered rats, to values comparable with the control. The elevated concentration of malondialdehyde after administration of cadmium chloride only was also reduced in co-administration with Agnus castus aqueous leaf extract at different doses. Agnus castus leaves displayed a protective role against the toxicity of cadmium chloride in the testes of Wistar rats.