1. Context
Alzheimer’s disease (AD) is a progressive neurodegenerative disease, which leads to brain dysfunction, poor performance in memory, executive function, visuospatial abilities, language, planning, and abstract reasoning (1). According to estimates, 4% of people under 65 suffer from Alzheimer’s. Cognitive decline is the most critical problem in AD, and the dorsolateral prefrontal cortex (dlPFC) plays an essential role in cognitive function (2). Therefore, this area has been targeted in many studies to assess cognitive dysfunction in AD, which is discussed here. This review provided a literature overview on the effects of tDCS over the dlPFC on cognitive dysfunction induced by AD.
2. Evidence Acquisition
As mentioned, this paper reviews the effects of tDCS over the dlPFC on cognitive function in AD patients based on a non-systematic approach. The articles were searched in the Scopus-, Google Scholar-, and PubMed-based literature review that targeted AD using tDCS over the dlPFC and conducted before Sep 2021. The keywords included “Alzheimer’s disease” AND “Transcranial Direct Stimulation (tDCS),” “Cognitive Dysfunction” AND “tDCS,” “Dorsolateral Prefrontal Cortex (dlPFC),” AND “tDCS.” The data collection process focused primarily on brain stimulation methods, patient characteristics, presence and/or absence of cognitive symptoms, study design and experimental protocols, and quantification of stimulation parameters. Studies conducted using healthy subjects and animal models were excluded from this study.
2.1. Pathophysiology of AD
Previous studies have suggested several mechanisms underlying AD pathophysiology and AD-induced cognitive decline, including cholinergic deficits, formation/accumulation of neurotoxic substances, oxidative stress, neuroinflammation, and mitochondrial dysfunction (3). Amyloid beta (Aβ) accumulation, particularly soluble neurotoxic oligomers, affects tau hyperphosphorylation, oxidative stress, neuroinflammation, and mitochondrial dysfunction, progressing disease through their downstream molecular cascades. Neurofibrillary tangles (NFTs) form intracellular aggregations that slowly propagate in the hippocampal region, known as the golden hallmark of AD (4, 5). Even though these findings may explain the probable foundations of cognitive impairment in AD, the precise mechanisms behind the loss of synapses and neurons in AD remain to be elucidated.
2.2. Pharmacological and Non-pharmacological Treatments
Available pharmacological treatments for AD are currently ineffective, causing side effects such as nausea, diarrhea, vomiting, dizziness, and agitation (Table 1) (6). Considering such limitations of pharmacological treatments, most researchers may prefer recently developed non-pharmacological interventions such as brain stimulation.
| Drugs | Side Effects |
|---|---|
| Donepezil | Insomnia, diarrhea, dizziness, fatigue, dizziness, rhinitis, anxiety, sleep disturbances, rare seizures |
| Galantamine | Convulsions, anorexia, dizziness, tremor, confusion, depression, rare seizures, and rare syncope |
| Rivastigmine | Dizziness, fatigue, headache, rare severe vomiting with esophageal rupture, rare syncope |
| Memantine | Dizziness, headache, constipation, rare seizures |
| Tacrine | Dizziness, diarrhea, dyspepsia, myalgia, anorexia, seizures, dizziness, insomnia, hepatotoxicity |
Common and Rare Side Effects of Pharmacological Treatment of Alzheimer’s Disease
2.3. Brain Stimulation
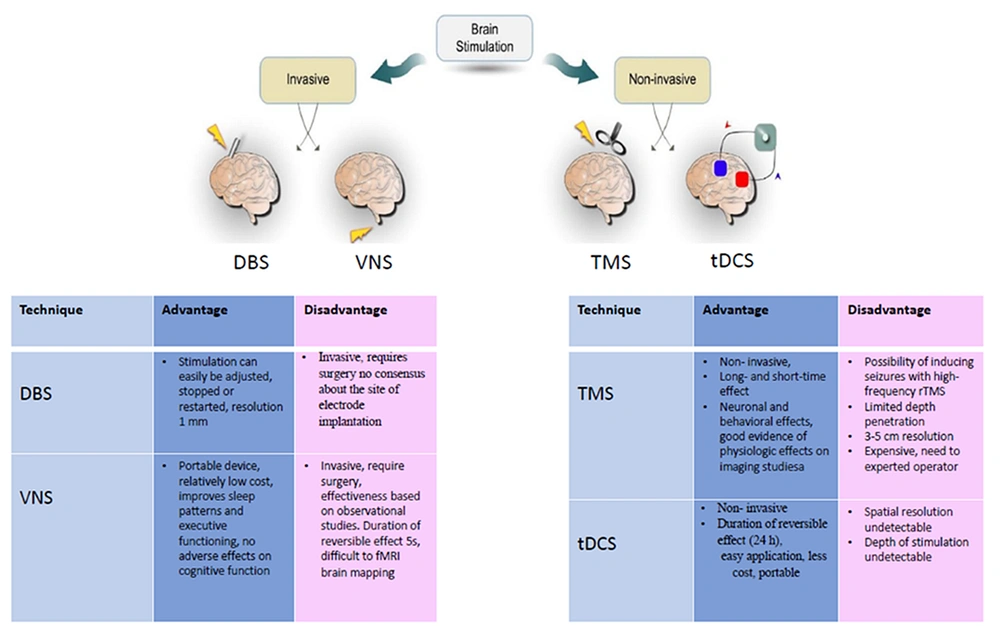
Brain stimulation modulates neuronal activity in specific brain regions or networks using an electronic or electromagnetic field. Invasive and noninvasive approaches are generally divided into two categories. There are two types of invasive brain stimulation: VNS and DBS. VNS can stimulate the vagus nerve using a programmable neurostimulator implanted subcutaneously in the chest or near the ear (7, 8). DBS involves implanted electrodes in the brain powered by a pulse generator placed subcutaneously in the chest (9). TMS and tDCS are two common forms of noninvasive brain stimulation. tDCS is a noninvasive approach that delivers weak electrical currents into the brain through saline-soaked sponge electrodes (10). TMS involves delivering magnetic currents via a coil of wire that generates an electric current in the targeted brain regions based on Faraday’s law (11).
These methods have some side effects which limit their application (see advantages and disadvantages in Figure 1). Among these methods, tDCS has the fewest side effects because it only induces mild effects like redness and itching of the skin (12). In addition, tDCS has several advantages over rTMS, such as being less invasive and expensive, easier to manage, more controllable, and portable, leading individuals to consider it as a suitable treatment at home in the future (13). Few studies have investigated the effects of tDCS on cognitive function in AD patients, given the efficiency of tDCS on various cognitive processes in healthy individuals as well as psychiatric and neurological patients, as mentioned above.
2.4. The dlPFC and Cognitive Function
The dlPFC is located in the middle frontal gyrus, typically defined as Brodmann areas 46 (BA 46) and 9 (BA 9) (14). This area is extensively connected to various cortical and subcortical regions involved in cognitive function, particularly in executive control and memory processing (15). More precisely, the dlPFC is involved in either working memory (16, 17), attentional bias (18), decision-making (19), language comprehension (20) and production (21), and cognitive control (22). Furthermore, some critical role is known for dlPFC, such as attention (23), problem-solving (24), working memory (25), and risky decision-making (26).
2.5. Effects of tDCS on Memory in AD Patients
Several studies have focused on the dlPFC due to its extensive connections to various cortical and subcortical regions involved in numerous cognitive functions (15). Numerous studies have evaluated the effects of tDCS over the left dlPFC on memory in AD patients. A single tDCS session has been shown to improve recognition memory (27), whereas multiple tDCS sessions in AD patients have improved global cognition (28). In contrast, Suemoto et al. demonstrated that repeated anodal tDCS applied over the dlPFC did not affect global cognitive performance (29).
The effect of tDCS over the dlPFC and recognition memory in AD improved visual recognition memory, but the Stroop digit span test showed no significant differences between the active and sham groups (27). Cotelli et al. (30) showed that anodal tDCS (AtDCS) plus computer-assisted training (CT) improved memory for naming faces in AD. This study showed that both (AtDCS + CT) and (PtDCS + CT) improved performance compared to the AtDCS + motor training group after two weeks of memory training (30). All patients received tDCS over the left dlPFC (five sessions of 25 min/day, at a constant current of 2 mA) for two weeks (30). A case study has examined the effect of 10 continuous daily sessions of tDCS over the left dlPFC and showed the stability of global cognition in mild AD subjects that lasted for three months (31). However, long-term treatment with tDCS over the dlPFC needs to be investigated. For example, a case study reported that daily tDCS stimulation over the temporal lobe for eight months improved memory and slowed cognitive decline in a patient with early-onset AD (32). Moreover, a recent study reported that applying tDCS at home could effectively improve cognitive decline accompanied by AD (33).
In the early stages of AD, patients suffer from dlPFC dysfunction (34), leading to WM impairment and especially its executive component (35). In addition, plasticity impairment in the dlPFC of AD patients should be considered an important index to improve WM deficits (36). Moreover, two trials have investigated noninvasive stimulation’s effect on improving WM in AD patients using tDCS in only one case (27, 31). Boggio et al. (27) applied tDCS over the left temporal and dlPFC to assess WM by a forward and backward digit span test and found no significant differences between sham and active stimulation (27), which may be due to the low sensitivity of the digit span test to the effects of tDCS (27). The electroencephalography (EEG) and event-related potentials used in another study have been found to improve the low sensitivity of previous tests using intelligent methods (37).
As discussed above, the effects of tDCS on memory have been explored in various studies, but the exact involved mechanisms are not yet clear. Alterations in multiple neurotransmitter systems may be one of the important mechanisms for the beneficial effects of tDCS (38). In addition, tDCS-induced modulation of neuronal excitability may, in part, account for some other beneficial effects of this technique in AD patients. For example, the temporal-parietal area in AD patients is hypoactive, and tDCS increases cortical activity (39). Cathodal tDCS may decrease hyperexcitability in the frontal region of AD patients (40). Despite these previous studies, the optimal stimulation protocol remains unknown, including current intensity, duration, session, and long-term period. Therefore, for future studies, the exact mechanisms involved in the effects of tDCS on memory, e.g., its probable effects on Ca2+ levels, brain-derived neurotrophic factor (BDNF), acetylcholine, dopamine, and other neurotransmitters, play important roles in neural plasticity.
2.6. The Effects of tDCS on Attention
Evidence shows that demanding executive processes of selective and divided attention may be impaired in the early stages of AD (35). AD is associated with deficits in span, focus, selective attention, and divided attention (41). As an important region in the attentional network, the PFC has been targeted in various tDCS studies. Boggio et al. (27) revealed that tDCS application over the left dlPFC in AD patients did not affect their attention in the Stroop task. In a literature survey, Siever (42) concluded that a large anodal electrode in FP1 or FP2 and a contralateral shoulder cathode might improve attention. Another protocol involves a large anodal electrode in FP1 or FP2 with a cathode in the neck. In stroke patients, experiments to strengthen attention have been performed using a similar protocol with an anodal electrode over the left dlPFC and a cathodal electrode in the contralateral supraorbital region (43). The effectiveness of tDCS over the dlPFC in AD patients has only been studied in a few studies, but data from other dementias suggest that stimulation can enhance attention. The main drawback of this approach includes using a bipolar montage (two electrodes were placed on the scalp) and the current distributions related to the size of the anodal and cathodal electrodes and the position of the reference electrode (10). However, the long-lasting effects of tDCS are poorly understood and need further investigation.
2.7. The tDCS and Decision Making
In AD patients, risky decision-making is associated with impairments in WM, mental flexibility, numerical ability, and inhibition, whereas impairments in learning ability, memory, and emotional processing occur in ambiguity (44). A literature survey revealed that a few studies have so far examined the effect of tDCS on the dlPFC on decision-making in AD patients (45, 46). Based on this study, the ability to decide in risky situations may be impaired, but ambiguity may not be damaged in AD patients (45). Thams et al. (46) evaluated the effects of the dlPFC on decision-making, combining three weeks of cognitive training (before and after brain stimulation) via tDCS (1 mA for 20 min). In this study, the immediate and long-term effects of tDCS (1 and 7 months after training) improved decision-making in AD patients (46). Overall, these studies have highlighted the need to assess other effects of tDCS over the dlPFC on decision-making in AD patients and determine the improvement of this noninvasive brain stimulation regarding the cognitive function component in AD patients. According to physiological theory, neurophysiological changes could be responsible for the altered decision-making in AD (47). Evidence reveals that alterations in various brain regions, including the frontal, parietal, and temporal cortices, and also changes in the neurotransmitters dopamine, serotonin, norepinephrine (48), and glutamate (49), could lead to altered decision-making in AD patients (47). More investigations are needed to examine the effects of tDCS on neurotransmitters and decision-making in AD patients.
3. Discussion
Alzheimer’s disease is a complex neurodegenerative disorder, representing one of modern society’s major problems. Research efforts to understand the underlying mechanisms of AD and develop treatment strategies have failed. Therefore, the efforts to elucidate the mechanism of the disease and find new methods continue with vigor.
This paper reviewed tDCS as a painless method to affect AD. This technique applies a weak electric current across the scalp whose distribution into cortical networks depends on various factors, including current density, stimulation duration, size and mounting of electrodes, and electric field orientation relative to the anatomical and geometric features. There are several possible explanations for these important issues. For instance, most studies above used a two-mA current intensity during a 10-session stimulation episode, but its duration varied from 20 to 30 min. Second, different electrode surfaces could be used in various cognitive functional networks, and a high-density tDCS (HD-tDCS) protocol may decrease this disturbance. Finally, duration and times of stimulations may be other determinant factors. Some previous studies have used daily stimulations, while others have applied 10 or 6 sessions during two weeks. Previous reports examined different protocols, and further efforts are required to ensure optimal protocols for tDCS application.
Most studies on the cognitive functions of tDCS in AD patients have primarily addressed multiple stimulations on the dlPFC, while a few have used single stimulations (50). tDCS may, in part, affect AD via inducing neural plasticity, especially LTP, in various brain regions. Most previous studies have followed up on the patients for six months. Since AD is a progressive disorder, further studies with longer durations of follow-up are recommended to assess the long-lasting effects of tDCS. The sufficient sample size in different cognitive domains, appropriate stimulation protocols (e.g., intensity, duration, and the number of repeated stimulations), cognitive tests with high sensitivity, and long-term follow-up would seem to be important issues in need of further investigations to evaluate the tDCS as a potential complementary treatment for AD.
AD is an irreversible, progressive neurodegenerative disease that begins decades before the onset of clinical symptoms (51). Previous studies have shown that changes in synaptic function, loss of synapses, and dystrophic axons are characteristic features in neurodegenerative diseases. Further studies have shown that Aβ and tau levels gradually increase. In contrast, symptomatic cognitive impairment occurs with a delay of 10 - 20 years between the onset of accumulation and a decrease in Aβ accumulation synchronous with the start of symptomatic disease (52). Knopman et al. (53) reported in a literature review that the Aβ and tau neurotoxicity leads to synaptic changes and synapse loss. In addition, studies have revealed a strong relationship between synaptic loss and cognitive decline (54). Moreover, evidence indicates that neuroinflammation occurs years before the onset of cognitive decline in AD (55). The anti-inflammatory effect of NA in the brain is well established (56), and the structure and functions of the NA system have also been shown to be disrupted at the early stages of AD. In animal models of AD, toxin-induced LC neurodegeneration could lead to increased accumulation of Aβ-plaques and neuroinflammation in the brain and alters the expression of NE receptors (57) as an early causal factor for AD.
Moreover, monoaminergic systems are involved in various physiological functions, including regulating movement, cognitive processes, mood, affective states, attention, and sleep. Monoaminergic systems also affect the degeneration of neurons within subcortical monoaminergic systems as an important early hallmark of AD before the deposition of extracellular β-amyloid plaques and intracellular NFT in the cortex (58). Evidence may also demonstrate that the initial degeneration of deep-seated monoaminergic nuclei may trigger the transneuronal spread of AD pathology toward the hippocampus and cortex, affecting learning and memory function (8, 59). The findings suggest exploring novel markers for therapeutic methods such as tDCS may be more effective before neuronal and synaptic dysfunction occurs.
4. Conclusions
Based on the results, tDCS can be an effective complementary therapy for AD-induced cognitive decline. The exact mechanisms involved in the beneficial effects of tDCS are not yet clear, but the probable mechanisms may include various neurophysiological signs and the underlying basis of AD, including reducing Aβ plaques and tau proteins, improving LTP and neural plasticity, and alterations of the abnormal neurotransmitter systems. Further studies should also consider important parameters, such as intensity, duration, and number of stimulations, in addition to the exact mechanisms. Furthermore, an important gap in previous findings is the lack of identifying biomarkers before neuronal and synaptic loss. Further studies are needed to develop a complete picture of the treatment strategy for AD and determine the above factors.