1. Background
Breast cancer is the most common malignancy in females, approximately one-third of all women’s tumors (1). Pathologically, the BC is classified into four subtypes luminal A, luminal B, HER-2+, and triple-negative breast cancer (TNBC) (2). Among these, the TNBC is known for the lack of estrogen (ER), progesterone receptors (PR), and growth factor receptor-2 (HER-2). The TNBC accounts for 12 - 20% of all BC cases. The incidence rate of TNBC increases in premenopausal women with more prevalence in younger (3).
Triple-negative tumors are characterized by aggressive behaviors with primary metastasis to the central nervous system, bones, lungs, and liver. Triple-negative tumors contain poor response to therapies, poor prognosis, and low survival rates compared to other BC subtypes (4). Unlike ER and HER-2+ cancers, TNBCs are highly resistant to current therapies because they are insensitive to endocrine and molecularly targeted treatments. Currently, only systemic chemotherapy and surgery are considered the main management for TNBC cases (5).
Over the thousands of years of human life, a large number of natural products of herbs have been used for the treatment of various diseases (6). In the 1950s, scientists systematically investigate natural organisms as a source of effective anticancer substances. Recently, it is proposed that the application of natural products is the most successful strategy for discovery of new anti-cancer drugs (7). Taraxasterol ((3β, 18α, 18α)-Urs-20(30)-en-3-ol)), as a pentacyclic triterpene, is isolated from Taraxacum officinale (TO) plant. Taraxacum officinale is used in traditional medicine for its milk-enhancing, choleretic, diuretic, and anti-inflammatory activities. Previous studies showed that TO extracts are effective in inhibiting proliferation and induction of apoptosis in cancer cells. According to the studies, taraxasterol is an important bioactive compound with strong anti-inflammatory and antioxidant activities (8).
The PI3K/AKT/mTOR signaling pathway plays an essential role in many cellular processes and is often altered in BC, leading to increased tumor growth. Small molecule inhibitors can effectively target the key elements in this pathway (9).
2. Objectives
Generally, development and evaluation of new agents against TNBC are difficult due to the high biological heterogeneity. Also, this type of tumor is considerably resistant to remedies which can potentially develop metastasis potential. Therefore, there is an urgent need for identification and development of new potential therapeutic drugs against TNBC with high efficiency and low side effects. Thus, the anticancer properties of taraxasterol on modulation of AKT/mTOR/β-Catenin signaling pathway (in TNBC cells) were investigated in the present laboratory-based study.
3. Methods
3.1. Taraxacum officinale Extraction and Taraxasterol Isolation
Taraxacum officinale was prepared by the Research Institute of Medicinal Plants (Tehran, Iran). The authenticity of the plant was confirmed by a herbalist. Then, the active substance of TO was extracted and purified under the supervision of the extraction company (Milad Herbal Medicine, Tehran, Iran). The desired extract was classified into different doses of 1, 2, 4, 8, 16, 32, 64, and 128 μM.
3.2. Cell Culture
The MDA-MB-231 cell line was obtained from the Pasteur Institute (Iran, Tehran). The cells were cultured (37°C, 5% CO2) in RPMI-1640 medium (Gibco, Germany) supplemented with 10% fetal bovine serum (FBS) (Gibco, Germany) and lack of antibiotics. All protocols of cell culture were considered carefully based on the previously published studies (10).
3.3. Laboratory Protocol
Following the preparation of various doses of TO, TNBC cells were cultured and exposed to TO. The procedures of exposure were applied based on the different times.
3.4. Cell Viability Assessment
2,5-diphenyl-2H-tetrazolium bromide (MTT) test was used to assess the cell viability. To this aim, 15 × 103 cells were cultured in each well of a 96-well plate and incubated overnight. Then, the cells were treated with various concentrations of taraxasterol (Sigma, Germany) for 24, 48, 72, and 96 h. The supernatant was discarded and MTT solution (Sigma, Germany) was exposed to each well (5 mg/mL in culture medium) for 3h. Following incubation, the supernatant was removed and formazan crystals were dissolved with 100 μL of DMSO. Optical density (OD) was measured at 570 nm with background subtraction at 690 nm. Data from three independent experiments were normalized using the following equation: Cell viability (%) = (OD sample value)/(OD cell control) × 100 (11).
3.5. Cell Apoptosis Investigation
The percentage of fragmented DNA following 24 h treatment with IC50 concentration of taraxasterol was calculated using diphenylamine assay. For the data report, the measurement of OD of samples was read by a spectrophotometer at 600 nm as described by Cohen and Duke (12).
3.6. Gene Expression Analysis
Gene expression assessment was performed by the real-time PCR method. Following the treatment of the cells with IC50 concentration for 24 h, the RNA was isolated from 1 × 106 cells of control and treatment cells using TRIZol reagent (Invitrogen, USA), and RNA purity and integrity were evaluated using a Nanodrop machine. Complementary DNA (cDNA) synthesis was carried out using a cDNA synthesis kit (Vivantis Technologies, Malaysia) and a real-time PCR process was performed by SYBR Premix Ex Taq Technology (TaKaRa Bio Inc., Japan). All laboratory processes were conducted according to the manufacturer’s instructions using the Applied Biosystems StepOne Real-Time PCR System. Changes were obtained based on the comparative method of Ct (2-ΔΔct) (13). GAPDH was used as an internal control and the primer sequences of various genes (HPRT, OPN, AKT1, mTOR, PTEN, β-Catenin, and GAPDH) were designed by GeneRunner software (v.3.05) according to Table 1.
| Genes | Sequences |
|---|---|
| HPRT | F: 5´-TGGACAGGACTGAACGTCTTG-3´ |
| R: 5´-CCAGCAGGTCAGCAAAGAATTTA-3´ | |
| OPN | F: 5´-ACCCTTCCAAGTAAGTCCAACG-3´ |
| R: 5´-GGTGAGAATCATCAGTGTCATCTAC-3´ | |
| AKT1 | F: 5´-AGCGACGTGGCTATTGTGAAG-3´ |
| R: 5´-GTACTCCCCTCGTTTGTGCAG-3´ | |
| mTOR | F: 5´-AACTCCGAGAGATGAGTCAAGA-3´ |
| R: 5´-AGTTGGTCATAGAAGCGAGTAGA-3´ | |
| PTEN | F: 5´-TGGATTCGACTTAGACTTGACCT-3´ |
| R: 5´-TTTGGCGGTGTCATAATGTCTT-3´ | |
| β-catenin | F: 5´-TACCTCCCAAGTCCTGTATGAG-3´ |
| R: 5´-TGAGCAGCATCAAACTGTGTAG-3´ | |
| GAPDH | F: 5´-TCCCTGAGCTGAACGGGAAG-3´ |
| R: 5´-GGAGGAGTGGGTGTCGCTGT-3´ |
3.7. Statistical Analysis
Following data extraction, the Kolmogorov-Smirnov test was the assessment of normal data distribution. One-way analysis of variance (one-way ANOVA) was used for statistical analysis and the Tukey post hoc test was conducted to determine the difference between the groups. Statistical Package for the Social Sciences 16 (SPSS Inc., Chicago, IL) was used for data analysis, and the results were expressed as Mean ± standard error. A P < 0.05 was also considered statistically significant (14).
4. Results
4.1. Taraxasterol and Triple-Negative Breast Cancer Cell Viability
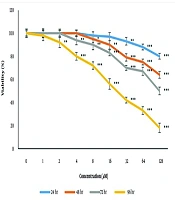
Following 24, 48, 72, and 96 h of treatment with various concentrations of taraxasterol, the TNBC cell viability was decreased significantly (P < 0.05) in the concentration- and time-dependent manner. These time and concentration dependencies were 24h of taraxasterol exposure with concentrations of 32, 64, and 128 μM, 48 h of taraxasterol exposure with concentrations of 8, 16, 32, 64, and 128 μM, 72 h of taraxasterol exposure with concentrations of 4, 8, 16, 32, 64, and 128 μM and 96 h of taraxasterol exposure with concentrations of 2, 4, 8, 16, 32, 64 and 128 μM which caused low TNBC cells viability. Also, the IC50 of taraxasterol was detected at 439.37 ± 6.8, 213.27 ± 5.78, 121 ± 7.98 and 27.86 ± 9.66 μM respectively after 24, 48, 72, and 96 h of exposure. These findings approved that the viability of TNBC cells decreases dramatically following exposure to taraxasterol. Also, it was concluded that by the acceleration of the taraxasterol dose and the time of exposure, the Viability Index decreases significantly (Figure 1).
4.2. Taraxasterol and Triple-Negative Breast Cancer Cells Apoptosis
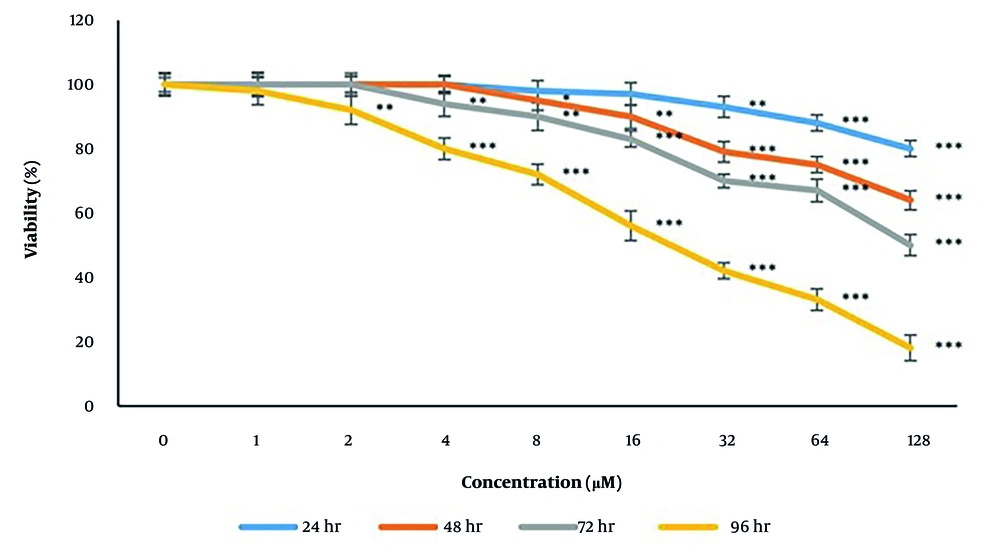
According to the data analysis, taraxasterol represented apoptotic features (44.02 ± 2.3% vs 1.01 ± 0.002% respectively in treatment vs control groups) significantly (P < 0.05) after 24 h at the IC50 concentration (439.37 µM) (Figure 2).
4.3. Taraxasterol and Gene Expression in Triple-Negative Breast Cancer Cells
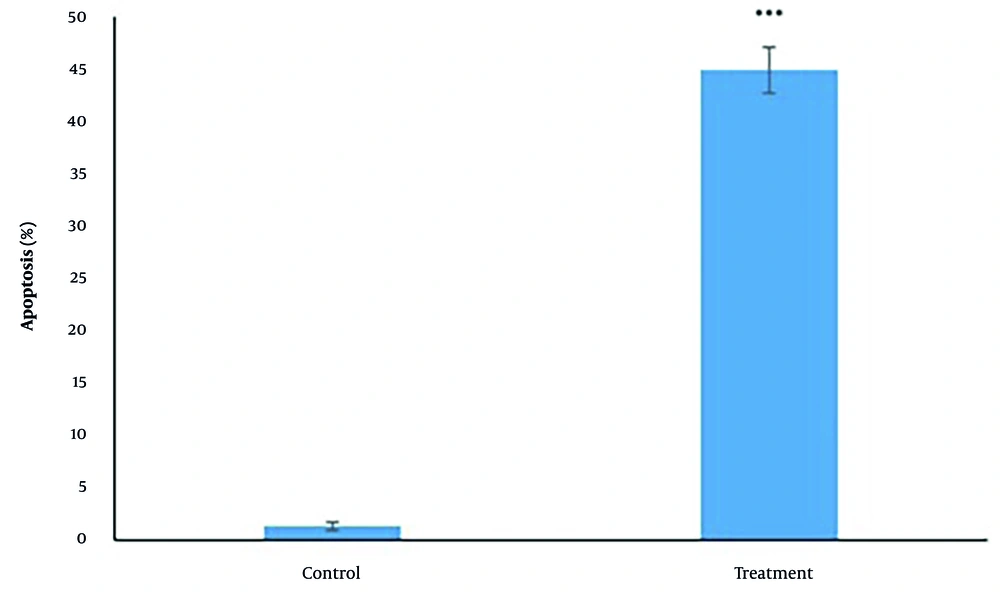
Following 24 h of treatment with IC50 concentration (439.37 µM) of tarxasterol, a significant (P < 0.05) decrease was detected in the expression of HPRT (0.72 ± 0.2 in treatment group than 1 in control group), OPN (0.49 ± 0.1 in treatment group than 1 in control group), AKT1 (0.85 ± 0.1 in treatment group than 1 in control group), mTOR (0.73 ± 0.1 in treatment group than 1 in control group) and β-catenin (0.43 ± 0.11 in treatment group than 1 in control group) genes. It can be concluded that the gene expression of HPRT, OPN, AKT1, mTOR, and β-catenin was decreased respectively by 24%, 29%, 12%, 55%, and 53% following tarxasterol exposure. PTEN (1.08 ± 0.22 in the treatment group than 1 in the control group) gene expression was also increased significantly (P < 0.05) by 1.08 after tarxasterol exposure (Figure 3).
5. Discussion
The TNBC is characterized by poor prognosis and resistance to conventional therapies. Phytochemicals such as taraxasterol, a bioactive compound from TO, have demonstrated anticancer properties. The current study confirms that taraxasterol induces apoptosis and reduces viability in MDA-MB-231 cells.
Taraxacum officinale antitumor activity was initially reported in 1981 (15). It exhibits cytotoxicity by promoting TNF-α and IL-1α production (16), key cytokines in apoptosis induction (17). Apoptosis is mediated via both extrinsic (death receptor) and intrinsic (mitochondrial) pathways. Taraxacum officinale also inhibits cancer cell invasiveness by attenuating phosphorylation of focal adhesion kinase (FAK) and Src, and reducing matrix metalloproteinases (MMP-2/9) activity (18).
Sox2, a pluripotency-associated transcription factor, is aberrantly expressed in multiple malignancies and facilitates proliferation and tumorigenesis, positioning it as a potential therapeutic target (19, 20).
RARβ2 encodes a nuclear receptor involved in retinoic acid-mediated transcriptional regulation, critical for morphogenesis and differentiation. Its expression is often suppressed in cancer, implicating its role as a tumor suppressor (15, 21).
TNF-related apoptosis-inducing ligand (TRAIL) is a selective inducer of apoptosis in cancer cells (22), although resistance mechanisms limit its efficacy. TIPRL inhibition sensitizes cells to TRAIL via MKK7-JNK pathway activation. Co-administration with TO enhances apoptosis through inhibition of the TIPRL-MKK7 interaction and JNK phosphorylation (15).
Taraxasterol markedly downregulated osteopontin (OPN), a multifunctional protein implicated in metastasis and poor outcomes in breast and other cancers (23). It also suppressed HPRT1 expression, a gene involved in nucleotide metabolism and highly expressed in TNBC. HPRT1 overexpression correlates with aggressive tumor phenotypes and regulates oncogenic pathways including Notch and ErbB, indicating its value as a biomarker and therapeutic target (24).
AKT1, commonly hyperactivated in BC (25), was downregulated following taraxasterol exposure, suggesting inhibition at both post-translational and transcriptional levels. mTOR expression was similarly reduced (26). PTEN expression, however, remained unchanged, aligning with prior findings where its modulation is context-dependent (27).
β-catenin, a key effector of Wnt signaling and regulator of cell adhesion and transcription, was also significantly reduced. Aberrant activation of β-catenin promotes oncogenesis by enhancing proliferation and invasion (28). Taraxasterol and similar plant-derived agents disrupt Wnt/β-catenin signaling and inhibit cancer stem cell function, thereby suppressing tumor progression (29).
Cytotoxicity analysis revealed that taraxasterol reduced cell viability in a time-dependent manner, with IC50 values declining from 439.37 ± 6.8 μM at 24 h to 27.86 ± 9.66 μM at 96 h (P < 0.05), indicating cumulative toxicity. Apoptosis induction was confirmed via diphenylamine assay. Gene expression profiling showed significant downregulation of HPRT1, OPN, AKT1, mTOR, and β-catenin at 24 h.
The AKT/mTOR/β-catenin signaling axis exhibits extensive crosstalk with Wnt and Notch pathways (15, 18, 29). Wnt signaling enhances β-catenin stabilization and nuclear translocation, while AKT/mTOR potentiates its activity. Notch signaling further modulates β-catenin via NICD interactions, reinforcing proliferative and survival signaling. Therefore, simultaneous targeting of these interconnected pathways may offer an effective strategy for TNBC therapy.
5.1. Conclusions
Taraxasterol as a pentacyclic triterpenoid available in the TO herb can potentially exert anticancer features on TNBC cells. The process of anti-proliferation of taraxasterol occurred following two stages of reduced survival rate and increased apoptosis levels through disruption of AKT/mTOR/β-Catenin signaling pathway. Since the administration of taraxasterol for cancer inhibition was found in a dose and time-associated manner, it is recommended to design an animal study for a deep assessment of the treatment.
5.2. Limitations
Although the plant authenticity was confirmed and extraction supervised, potential variability in the purity and composition of taraxasterol extract may affect reproducibility and consistency of results, especially since detailed characterization (e.g., purity percentage, batch-to-batch variation) is not described. The study relies solely on the MDA-MB-231 TNBC cell line, which may limit the generalizability of findings across other TNBC subtypes or BC models with different genetic backgrounds. Apoptosis was assessed only by diphenylamine assay measuring DNA fragmentation at 24 hours, without complementary methods (e.g., flow cytometry, caspase activity) to confirm apoptotic pathways and kinetics.