1. Background
Alzheimer's disease (AD) is one of the most prevalent neurodegenerative diseases leading to dementia in the elderly (1). The loss of neurons results in cognitive disorders, behavioral disturbances, memory dysfunctions, and, at advanced stages, physical disabilities and death (2). The formation of amyloid β (Aβ) plaque aggregates and tau protein tangles are primary causes of AD (1), promoting oxidative stress, a leading cause of neuronal toxicity and death (3). Another critical aspect of AD pathogenesis is the overactivation of glutamate N-methyl-D-aspartate (NMDA) receptors, leading to memory loss and learning difficulties (4). Therefore, inhibiting these receptors could be beneficial in alleviating AD symptoms. Memantine hydrochloride, an uncompetitive antagonist of NMDA receptors, has proven effective in slowing AD progression and improving symptoms through its anti-glutamatergic properties (5). Given the multifactorial nature of AD, multifunctional therapeutic approaches that enhance the patient's quality of life are highly advantageous (6). In recent decades, healthcare scientists have garnered significant interest in nanotechnology in drug delivery and medicine (7). Fullerene C60, a carbon allotrope with a nanoscale diameter (0.7 nanometers) and a hollow spherical shape, possesses unique physicochemical properties (8). Thanks to its structure rich in double bonds, this nanoparticle is an efficient radical scavenger, making it a candidate for treating neurodegenerative disorders like AD (9). Some studies have suggested that water-soluble formulations of fullerene can prevent the aggregation of Aβ plaques (3) and thereby inhibit the cell apoptosis process that follows plaque aggregate formation (10). Additionally, it has been proposed that fullerenes can reduce the activity of NMDA receptors, although they are not used as NMDA antagonists (9).
Given these characteristics, administering an aqueous suspension of fullerene (C60) in a rat model of AD is anticipated to be highly beneficial for evaluating whether its multifaceted activity can alleviate the disease's symptoms.
2. Methods
2.1. Chemicals
Fullerene (C60) 99.5% and anhydrous toluene (99.8%) were acquired from Merck (Germany). Acetonitrile (HPLC grade) was sourced from Scharlau (Spain). Memantine hydrochloride was graciously provided by Sobhan Darou Co. (Iran). Wistar rats were procured from the Pasteur Institute (Iran). Scopolamine hydrobromide trihydrate (≥ 98%) was purchased from Sigma-Aldrich (India).
2.2. Nano Formulation Preparation
In line with prior studies, a fullerene aqueous suspension (FAS) was prepared (11, 12). Briefly, fullerene (C60) was dissolved in anhydrous toluene at a concentration of 3 mg/mL, producing a dark purple solution. This solution was then mixed with deionized water in a 3:1 ratio. The mixture was stirred for 24 hours at room temperature in a dark setting, after which the toluene was fully evaporated using ultrasonic treatment for several hours under a fume hood. This process resulted in the disappearance of the purple color, leaving behind a pale yellow solution. The final formulation was stored at temperatures between 2 and 8°C in the dark.
To ensure the absence of toluene residue in the formulation, a GC-MS test was conducted, revealing no toluene peaks (Agilent Technologies, 5975C). The size distribution and zeta potential of C60 nanoparticles dispersed in water were determined at 25°C using the dynamic light scattering (DLS) technique (Brookhaven Instruments). The concentration of C60 nanoparticles in water was measured using high-performance liquid chromatography (HPLC) under the following conditions: Isocratic separation was achieved using a Knauer Smartline 2500 HPLC system with a Eurosphere II -C18 column (250 × 4.6 mm, 100 - 5). The mobile phase consisted of toluene and acetonitrile (60:40, v/v), with a flow rate of 1 mL/min and ultraviolet detection at 328 nm.
2.3. Stability Test
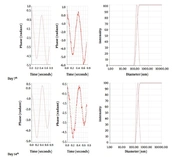
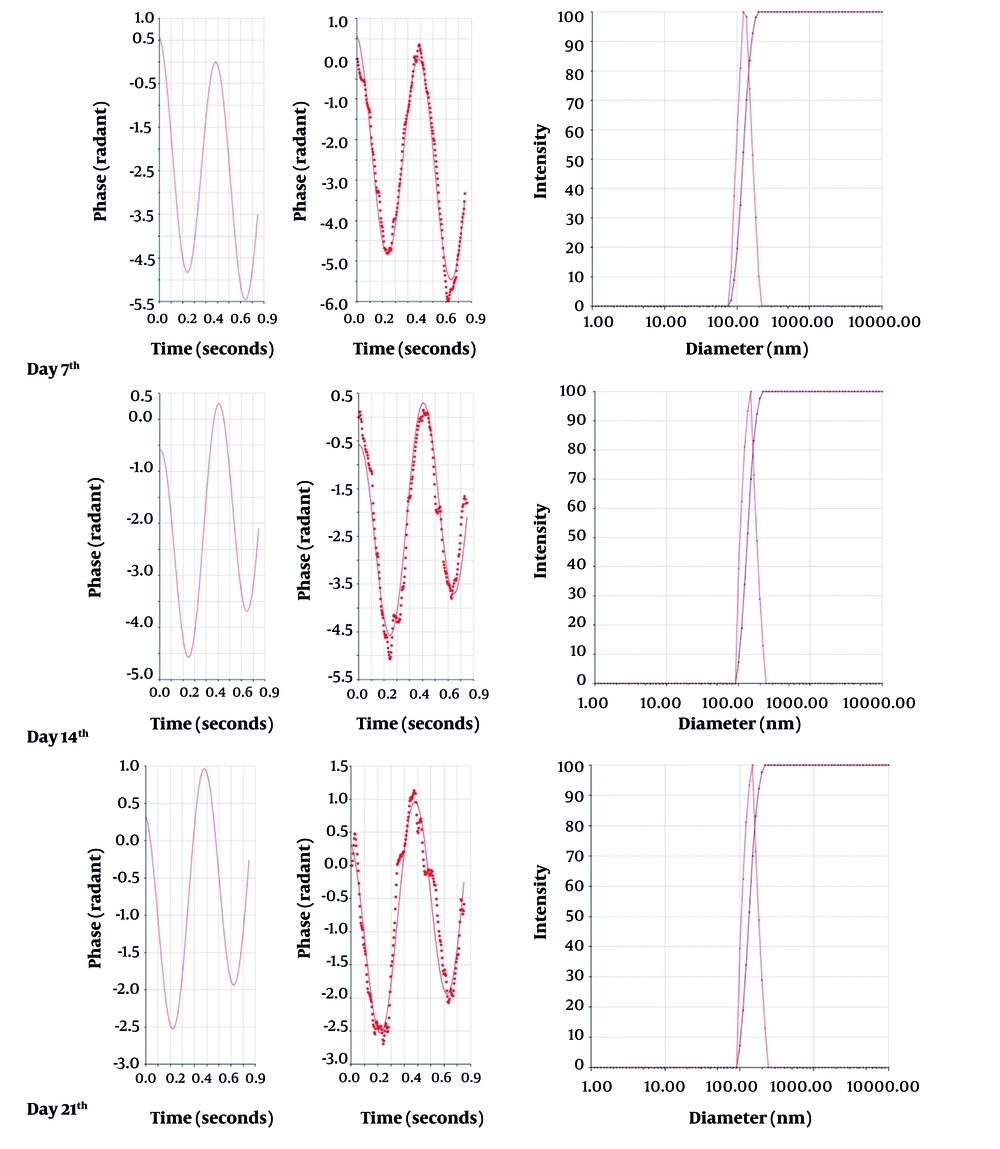
A stability test was conducted over 21 days, with assessments every 7 days, to monitor appearance properties such as color and clarity, as well as particle size and zeta potential of the nanoparticles. During each assessment period, zeta potential measurements were taken in duplicate, and size measurements were taken in triplicate for each sample (Figure 1).
2.4. Animals
A total of 24 male Wistar rats, weighing between 200 and 240 g, were randomly divided into four groups:
(1) Control group: Received normal saline (i.p.) for 10 consecutive days;
(2) Scopolamine-treated group: Given scopolamine HBr (2 mg/kg, i.p.) for 10 days;
(3) Memantine-treated group: Administered scopolamine HBr (2 mg/kg, i.p.) followed by memantine HCL (10 mg/kg, i.p.) for 7 days;
(4) Fullerene-treated group: Received scopolamine HBr (2 mg/kg, i.p.) for 10 days, followed by a colloidal solution of fullerene C60 (21 µg/mL, 1 mL, BID) via i.p. injection for 7 days. Training was conducted on days 18, 19, and 20, and on the 21st day, the rats were tested to determine if they could locate the platform.
The rats were housed under standard cage and laboratory conditions (i.e., temperature at 23 ± 2°C, humidity at 50 ± 10%, and a 12-hour light-dark cycle), with access to water and food.
2.5. Behavioral Learning
To conduct the Morris water maze test, the tank was divided into four equal quadrants (Q1 - Q4), with a platform placed in the second quadrant and the tank filled with water.
For each test, the software randomly selected a quadrant of the tank in which to place each rat. Scopolamine hydrobromide, a nonselective muscarinic antagonist, can simulate AD symptoms (13). To induce memory impairment, scopolamine was administered (2 mg/kg, i.p.) for 10 days to each rat. From the seventh to the ninth day after injection, the rats were placed in the MWM tank for training with a platform in the Q2 segment. On the 10th day, the platform was removed to assess if the rats could recall the platform's location and to measure the time they spent in the Q2 quadrant.
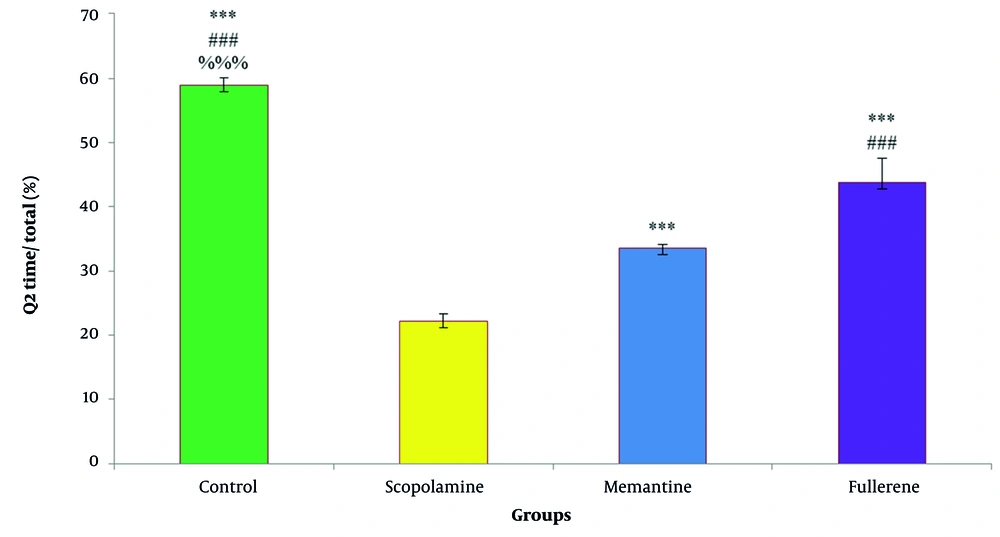
Their improvement in spatial memory was evaluated based on this parameter (3). Figure 2 shows the comparison of the test groups, and Figure 3 displays the behavioral pattern graphs of the rats.
Influence of fullerene (C60) administration on spatial memory in rats. Fullerene (21 µg/mL, 1 mL, BID) and memantine HCL (10 mg/kg) were administered (i.p.) for 10 days after scopolamine i.p. injection (2 mg/Kg). Data are represented as mean ± SEM (n = 6 per group). * P < 0.05 vs. scopolamine, # P < 0.05 vs. memantine, and % P < 0.05 vs. fullerene.
2.6. Statistics
SPSS 16 was used for data analysis. One-way analysis of variance (ANOVA) and post hoc Tukey tests were conducted to compare groups and interpret the results. A P-value of less than 0.05 was considered significant.
3. Results
3.1. DLS Data Analysis
The results of the stability test are presented in Table 1. According to the figure, the average particle size, polydispersity index (PDI), and zeta potential were 119.1 ± 3.4 nm, 0.15, and -12.22 ± 6 mV, respectively.
| Formulation and Day | Color Change | Clarity Change | Average Particle Size, nm | Polydispersity Index | Zeta Potential, mV |
|---|---|---|---|---|---|
| Fullerene (FAS) | |||||
| 1 | Yellow | Clear | 116.3 | 0.13 | -19.6 |
| 7 | No | No | 116.7 | 0.16 | -13.7 |
| 14 | No | No | 121.6 | 0.14 | -8.3 |
| 21 | No | No | 122.0 | 0.15 | -7.3 |
3.2. Fullerene Suspension Calibration Curve
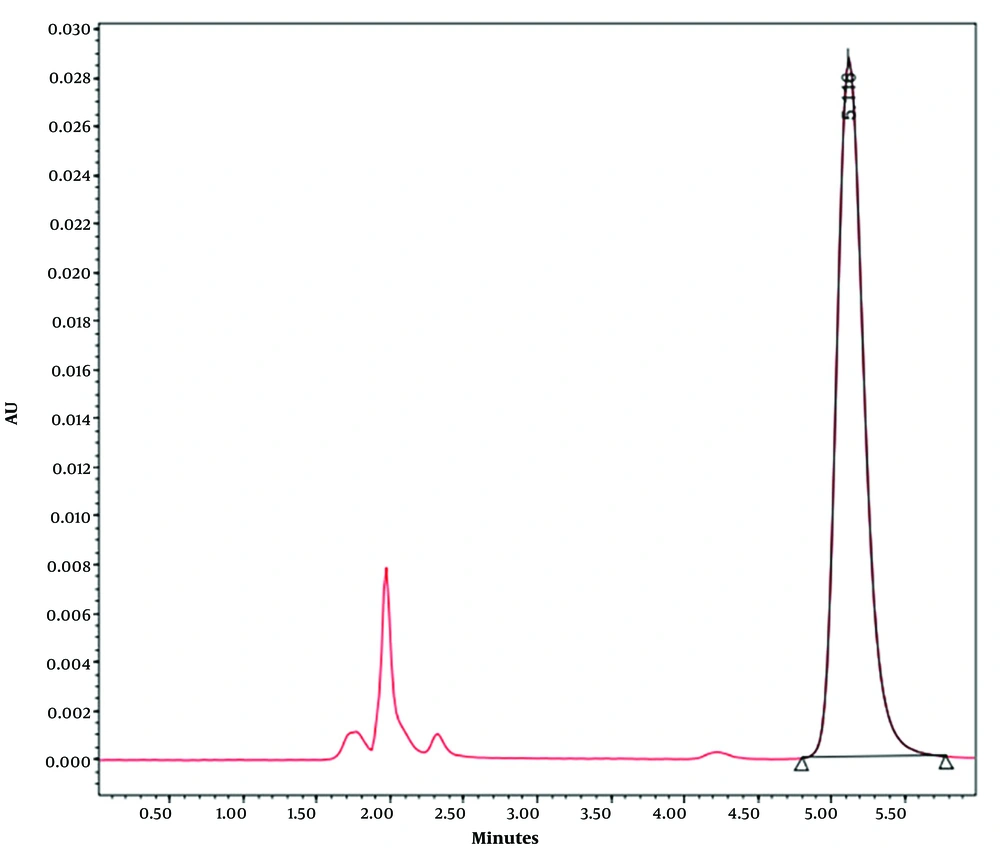
The calibration curve for the fullerene colloidal aqueous suspension was established following the HPLC procedure described earlier. Fullerene standard solutions ranged from 12.5 to 50 µg/mL in a toluene/acetonitrile mixture (60:40 V/V), producing a linear graph (Y = 93676.818X; n = 5; R2 = 0.9985) with a retention time of approximately 5 minutes (Figure 4). The limit of detection (LOD) and limit of quantification (LOQ) were 0.39 µg/mL and 1.3 µg/mL, respectively (Table 2).
| Sample | Concentration Range, µg/mL | Slope | Intercept | R2 | Concentration Points |
|---|---|---|---|---|---|
| Fullerene C60 | 12.5 - 50 | 93676.818 | - | 0.9985 | 6 |
3.3. HPLC Method Validation
Fullerene standard solutions of 12.5, 30, and 50 µg/mL were prepared in a toluene and acetonitrile mixture (60:40) in triplicates and analyzed in three separate runs. The coefficient of variation (CV%) was calculated for each experiment. The intraday and interday precision (relative standard deviation, RSD%) and accuracy of the HPLC method were determined using calibration curve standards.
3.4. Scopolamine Effect of Spatial Memory Suppression
Scopolamine hydrobromide (2 mg/kg) was administered intraperitoneally (i.p.) for 10 days before testing. Spatial memory was assessed by measuring the time rats spent searching for the platform in the Q2 segment. As indicated in Figures 1 and 2, the scopolamine hydrobromide-treated group spent less time in the Q2 section compared to the control, positive control, and fullerene-treated groups. This suggests that scopolamine may impair spatial memory.
3.5. Memantine HCL Effect on Spatial Memory Improvement
Memantine HCL (10 mg/kg) was administered (i.p.) for a continuous period of 7 days following scopolamine HBr (2 mg/kg) treatment. The effect of the drug on memory impairment was evaluated similarly to the scopolamine group (Figure 1).
3.6. Fullerene Ameliorating Effect on Spatial Memory
After 10 days of scopolamine administration (2 mg/kg), a colloidal solution of fullerene (21 µg/mL, 1 mL, BID) was administered (i.p.) for 7 days. For the following 3 days, rats were trained in the Morris water maze tank, and on the 21st day, they were tested to determine any improvement in memory. According to Figure 1, the fullerene-treated group exhibited significantly better results than the memantine-treated group.
4. Discussion
The FAS has been shown to be highly stable from a physicochemical perspective (14). In our study, the formulation demonstrated satisfactory stability over a 21-day period, as evidenced by the average particle size and zeta potential of the nanoparticles dispersed in water. The zeta potential of the nanoparticles remained relatively unchanged throughout the testing period, whereas there was a significant change in particle size (Table 1). This alteration could be attributed to the oxidation process of fullerene. Numerous studies have highlighted that water-soluble fullerenes are potent free radical scavengers reacting with reactive oxygen species (ROS). Takada et al., in 2006, conducted extensive research and elucidated this property (15, 16). Therefore, it can be inferred that the increase in zeta potential might result from the addition of positive hydrogen ions to the double bonds of fullerene during the oxidation process, not only making the zeta potential more positive but also leading to an increase in the size of fullerene particles. However, as indicated by previous research, another contributing factor to this phenomenon is the extreme hydrophobicity of fullerenes, which causes them to aggregate and form clusters, in addition to the presence of individual C60 fullerenes (12, 17, 18).
Glutamate is a fundamental neurotransmitter in the central nervous system (CNS) (19). The glutamatergic system, located in the pyramidal neurons of the hippocampus and cortex, plays a crucial role in cognitive processes, memory, and learning by initiating cytosolic calcium signals (20). In AD, the incidence of pyramidal neurons in the brain is lower than in a normal and healthy condition, indicating a hypoactivity of the glutamatergic system in this disease (19). However, the hyperactivity of glutamate NMDA receptors can lead to several undesirable outcomes, such as severe depolarization of the postsynaptic membrane, calcium overload in mitochondria, dysfunction in mitochondrial operations for expelling oxygen free radicals (ROS) and triggering oxidative stress. All these factors contribute to cell apoptosis and neurodegeneration (21, 22).
Fullerenes and their water-soluble derivatives have demonstrated strong antioxidant activities, the ability to inhibit neuronal cell apoptosis, and the capacity to block glutamate receptors (23, 24). As potent radical scavengers, they can effectively contribute to the treatment of neurodegenerative diseases (25, 26).
In this study, the effect of water-soluble pristine fullerene (C60) was examined and compared with the effect of memantine hydrochloride on AD. Memantine is known to improve cognitive disorders in AD through the blockage of NMDA glutamate receptors (27). As shown in Figure 1, the administration of fullerene significantly enhanced cognitive behavior in rats compared to those treated with scopolamine and memantine. This phenomenon is likely related to the antioxidant activity of fullerene as well as its anti-glutamatergic effect (23, 26).
Despite the promising results obtained in this study, a major limitation was the low concentration of fullerene particles dispersed in water. It is anticipated that increasing the yield of the FAS formulation could lead to even better outcomes.
4.1. Conclusions
The findings from this research suggest that the aqueous suspension of fullerene is a highly stable formulation, and its administration can significantly benefit the improvement of cognitive behavior and spatial memory in rats afflicted with AD.