1. Context
Dermatological manifestations of diabetes mellitus are common but often remain neglected, due to the heterogeneity of clinical signs, as well as the need for an interdisciplinary approach. Currently, diabetes is becoming more prevalent in developed countries (1). According to the WHO report, the number of people with diabetes worldwide increased four times in 25 years, from 108 million in 1980 to 422 million in 2014 (2). Diabetes may lead to serious consequences like blindness, renal failure, heart attack, stroke, and lower limb amputation if treated inadequately (1, 2). Approximately 1.6 million patients died in 2015, due to the complications of diabetes and 51% to 97% of diabetic patients presented with skin manifestations at the same time (2, 3). These facts highlight the importance of dermatologists for early detection of diabetes.
This review aims to highlight the pathogenetic mechanisms and clinical picture of skin conditions, associated with diabetes mellitus.
2. Evidence Acquisition
Skin manifestations of diabetes mellitus are related to increased glucose levels. Hyperglycemia affects skin homeostasis by inhibiting keratinocyte proliferation and phagocytosis, and inducing endothelial cell apoptosis. Later in the disease course, the involvement of the peripheral nervous system and vascular changes (micro and macroangiopathy) become the leading factors (3). Diabetic neuropathy is subdivided into sensory, motor, and autonomic forms. Sensory neuropathy is responsible for itching and paresthesia, as well as the reduced sensation of the skin. Motor neuropathy leads to changes in muscle tonus, causing foot deformities and development of the famous diabetic Charcot’s foot (4). Alterations in the autonomic nervous system can change sweat secretion, aggravating skin dryness and cause xerosis, hyperkeratosis, fissure formation, and compromised skin vascularization, resulting in diabetic dermopathy and sclerotic skin changes (5).
3. Results
There are different classifications of skin changes in diabetes, depending on the frequency (common or infrequent), the onset (early or late), and the type of diabetes. Skin manifestations are more common in insulin-dependent diabetes mellitus than in the insulin-independent variant (Box 1). As the majority of dermatoses occur in both types of diabetes, the most useful classification from the practical point of view is as follows: (1) cutaneous manifestation specific only to diabetes; (2) compatible dermatoses not specific to diabetes; (3) skin infection associated with diabetes; and (4) skin manifestation due to anti-diabetic therapy (6).
| Skin Manifestations on the Type of Diabetes Mellitus |
|---|
| Type I |
| Bullosis diabeticorum |
| Necrobiosis lipoidica |
| Keratosis pilaris |
| Acquired ichthyosis |
| Vitiligo |
| Type II |
| Diabetic dermopathy |
| Acanthosis nigricans |
| Granuloma annulare |
| Scleredema adultorum (Buschke) |
| Erythema faciale |
3.1. Cutaneous Manifestation Specific to Diabetes
Typical skin manifestations are largely associated with diabetes mellitus, although they may have a different etiology and pathogenesis in some patients. These dermatoses indicate either new-onset diabetes or poor glycemic control in already diagnosed diabetes.
3.1.1. Diabetic dermopathy
Diabetic dermopathy is a common skin manifestation seen in more than half of diabetic patients. It is more common in men after the fifth decade of life. It occurs in the later stages of diabetes, often combined with nephropathy, neuropathy or retinopathy (7). Clinically, it presents with the appearance of well-demarcated hyperpigmented atrophic plaques on the anterior surface of the legs (7, 8). The pathogenesis of the disease includes trauma or peripheral angiopathy with subsequent accumulation of melanin and hemosiderin in the dermis. Diabetic dermopathy frequently associates with renal atherosclerosis (7). Differential diagnosis with skin manifestations of chronic venous insufficiency or nummular dermatitis have to be made.
3.1.2. Diabetic Foot Syndrome
Diabetic foot syndrome (Malum perforans) is a relatively common disease, affecting about 6% of the population (9). It occurs in 15% - 25% of diabetic patients, a little more often among those suffering from insulin-independent diabetes (10). Clinically, Malum perforans presents with hyperkeratosis, xerosis and callosity, which subsequently develops to an ulcer with callous edges and a necrotic, non-healing bottom (10). The pathogenesis of the disease is complex. It combines both chronic trauma and ischemic, neuropathic changes caused by diabetes (4). In the diabetic foot syndrome, the ulcer usually occurs on the skin overlying bony prominences, commonly over the third tarsometatarsal joint of the foot.
Patients with insulin-dependent diabetes and diabetic foot have more tendency towards infectious complications like gangrene and osteomyelitis, which may impose foot amputation (9). The development of diabetic neuro-osteoarthropathy, progressive bone and joint deformation, and the destruction of the foot, are described in the literature as the Charcot foot syndrome (11). The diabetic foot can also be combined with erythromelalgia with erythema, burning and pain on the foot provoked by warming. The differential diagnosis includes osteopathy with a different genesis: syringomyelia, alcoholic neuropathy, termed Acropathia ulcero-mutilans acquisita (Bureau-Barriere), etc. (12).
The treatment of the diabetic foot is complex and requires the following measures: chronic trauma prevention, daily hygiene, antiseptics/antibiotics inhibiting bacterial and mycotic colonization, hydrogels, epithelotonic agents, hyperbaric oxygen therapy, growth factors and surgery (4, 9).
3.1.3. Acanthosis Nigricans
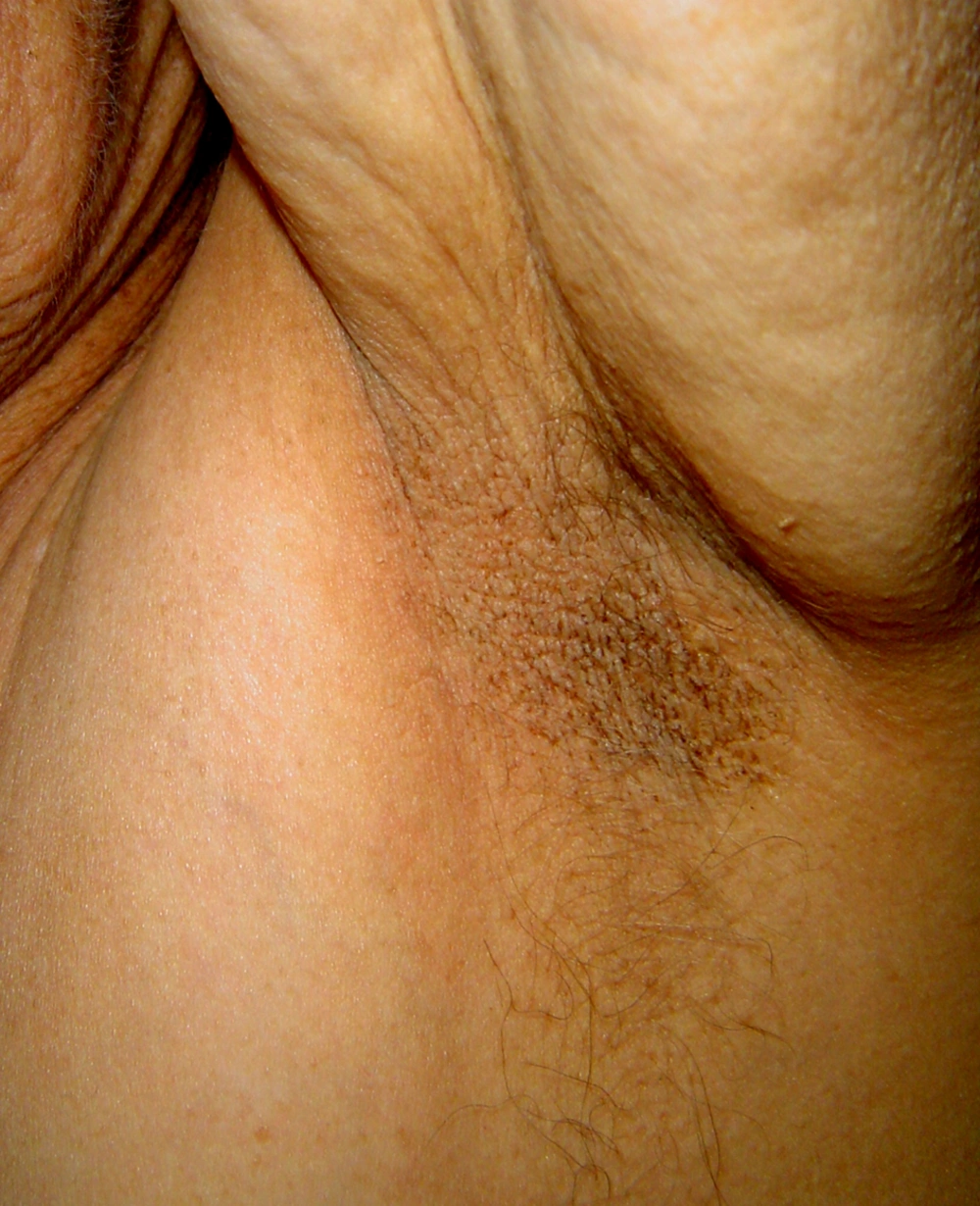
Acanthosis nigricans is a dermatosis more common in insulin-independent diabetes, but also observed in patients with obesity, acromegaly, Cushing syndrome or neoplastic diseases (13). Hyperglycemia is found in more than half of the patients (14). It presents clinically with hyperpigmented verrucous linear plaques, mainly affecting skin folds (axillae, inguinal folds, and neck) (Figure 1). The role of insulin as an activator of IGF-1 receptors in keratinocytes and dermal fibroblasts is discussed in the pathogenesis of the disease (15).
3.1.4. Scleroderma-Like Skin Changes
Scleroderma-like skin changes include a heterogeneous group of syndromes with a common clinical presentation of skin tightening. Progressive finger skin thickening affects 8% - 50% of diabetic patients and is more common in type I diabetes mellitus (16). The changes resemble sclerodactyly in systemic sclerosis, lacking both respiratory and gastrointestinal tract involvement and without autoantibodies in the serum. However, sclerodactyly in diabetes is combined with a high risk of diabetic nephropathy and retinopathy. Diabetes is also common in Dupuytren’s contracture and stenosing tenosynovitis with the “trigger finger” symptom (17). Another important symptom is the “prayer sign” in which the patient is unable to press his palms tightly due to the tightening and flexion contracture of the fingers (18).
3.1.5. Scleredema Adultorum
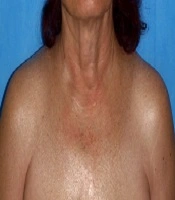
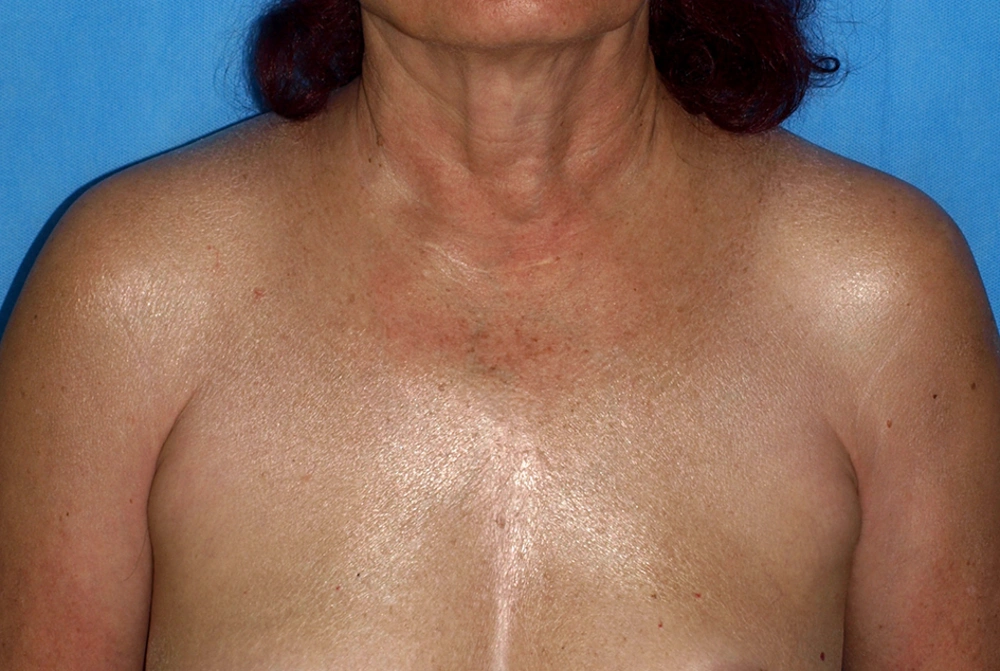
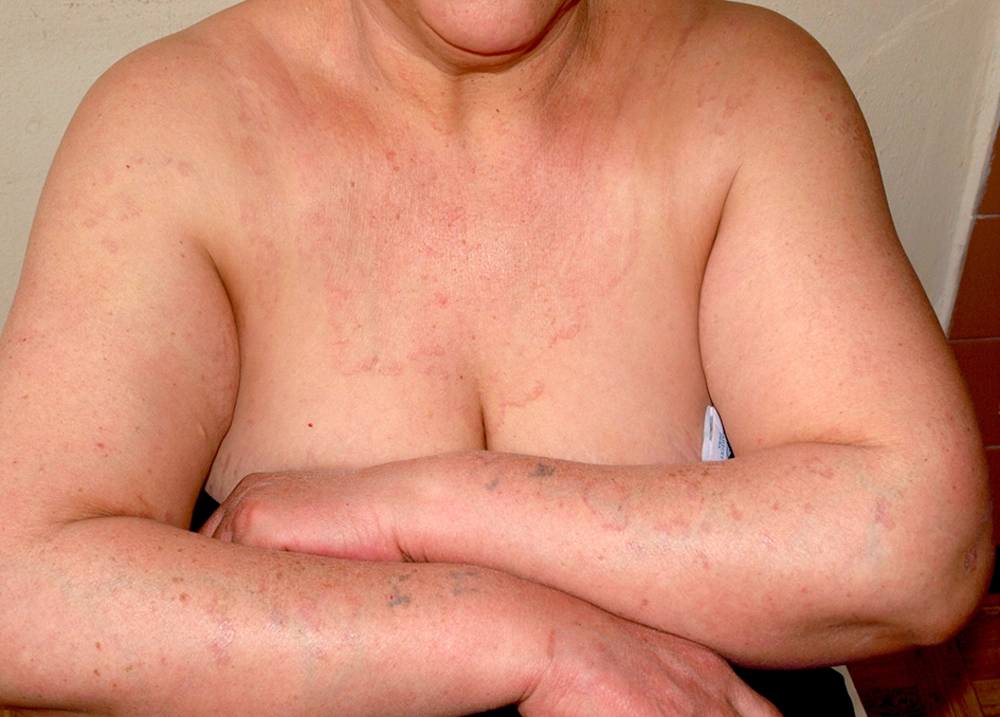
Scleredema adultorum (Buschke) is a rare disease with a multi-etiological origin, first described in 1902 (19). There are three variants of scleredema: (1) scleredema in young or middle-aged women, occurring after infectious diseases; (2) slowly advancing scleredema in patients with monoclonal gammopathies/multiple myeloma; and (3) scleredema associated with diabetes mellitus (19). Only 2% - 15% of patients with diabetes develop scleredema adultorum. The disease is more common in obese, middle-aged men with uncontrolled glycemia. The ratio of insulin-dependent to insulin-independent diabetes is 6.5/1 (20). Clinically, the disease presents with erythema and skin thickening, covering mainly the chest, slowly progressing to the back of the neck, shoulders, face, and upper extremities (Figure 2). A case with symmetric periorbital edema and partial vision blockage was also described (21). Scleredema adultorum is usually treatment-resistant and the correction of hyperglycemia rarely ameliorates the condition.
3.1.6. Necrobiosis Lipoidica Diabeticorum
Necrobiosis lipoidica diabeticorum is an inflammatory granulomatous dermatosis with collagen degeneration and vascular involvement. The disease is more common in women after the age of 30. Necrobiosis lipoidica often associates with insulin-independent diabetes and does not correlate with glycemic control. It clinically manifests with symmetrical, yellow-brown scleroatrophic plaques, located on the anterior surface of the shins, tending to ulcerate in one-third of cases (Figure 3). In rare cases, skin lesions can affect the trunk, upper extremities, and even the scalp (22).
Necrobiosis lipoidica diabeticorum perforans in a 42-year-old patient with uncontrolled diabetes 22
3.1.7. Bullosis Diabeticorum
Bullosis diabeticorum is a rare dermatological manifestation, affecting less than 0.5% of patients with diabetes. It was initially described by Kramer in 1930 and reported twice as often in males, between the ages of 50 and 70 (23). Clinically, patients present with tense sterile bullae that appear suddenly on unaltered skin, usually on the limbs and less often on the trunk (8, 23). The typical location raises the question of whether microtraumas take part in the pathogenesis of the disease. Usually, subjective symptoms are lacking. The lesions regress spontaneously for two to six weeks but can recur. Differential diagnosis is made with a bullous reaction from physical factors, bullous pemphigoid or porphyria.
3.2. Compatible Dermatosis not Specific only to Diabetes
This group includes skin manifestations often seen in several metabolic diseases, not exclusively diabetes.
3.2.1. Diabetes-Associated Pruritus
Diabetes-associated pruritus can be both disseminated and localized, most commonly on the scalp, ankles, inguinal folds, and genitalia. It may be a sign of skin infection or xerosis, but more often appears in unaffected skin (24).
3.2.2. Xerosis Cutis
Xerosis cutis occurs in about 44% of patients, considered one of the earliest manifestations of type I diabetes mellitus (25). Dry skin, however, is also a symptom of atopic dermatitis, psoriasis, acquired or congenital keratoderma and inflammatory or oncological diseases, as well as a manifestation of skin aging. The differential diagnosis is very wide, which determines the low diagnostic value of dry skin as a clinical sign.
3.2.3. Acquired Ichthyosis
Acquired ichthyosis is a relatively infrequent disease, affecting up to 25% of patients with insulin-independent diabetes (26). In addition to diabetes, neoplasms, infections, autoimmune diseases, and endocrinopathies are also important in the etiology of the disease (26). Ichthyosis mainly affects the lower legs and ankles, with dry, pigmented, polygonal scales. The disease is a manifestation of rapid skin aging and hyperkeratosis due to decreased corneocyte desquamation.
3.2.4. Keratosis Pilaris
Keratosis pilaris is seen in about 20% of patients with diabetes. The condition is considered one of the minor criteria for atopy and is common in children and adolescents with atopic dermatitis (27). The skin lesions are located on the lateral surfaces of the upper limbs, hips and face and are represented with keratotic follicular papules on erythematous or pigmented skin (28). The treatment with emollients and topical keratolytics has been successful although with temporary effect in the majority of patients.
3.2.5. Huntley Papules
Huntley papules are skin-colored, hyperkeratotic papules, grouped as “pebbles” on the extensor side of fingers (29). They are associated with type II diabetes and sometimes precede sclerotic finger changes and the “prayer sign”. Topical treatment is difficult and often ineffective.
3.2.6. Rubeosis Faciei Diabeticorum
Rubeosis faciei diabeticorum is facial erythema, affecting about half of the hospitalized diabetic patients (30). Rubeosis is a manifestation of suboptimal glycemic control, and associates with venous hyperemia and microangiopathy. The differential diagnosis is made with rosacea, malar rash in systemic lupus erythematosus or contact dermatitis. The strict control of blood glycemia leads to clinical improvement.
3.2.7. Palmar Erythema
Palmar erythema is usually symmetrical and accompanied by an elevated local temperature. It affects about 4% of patients with diabetes mellitus (31). Unlike erythromelalgia, the patients do not have any subjective symptoms in this condition. Palmar erythema is a symptom of venous hyperemia and microangiopathy. Differential diagnosis includes neoplastic erythema, rheumatoid arthritis, thyrotoxicosis, hepatic damage, drug-induced erythema and erythema in pregnancy.
3.2.8. Granuloma Annulare
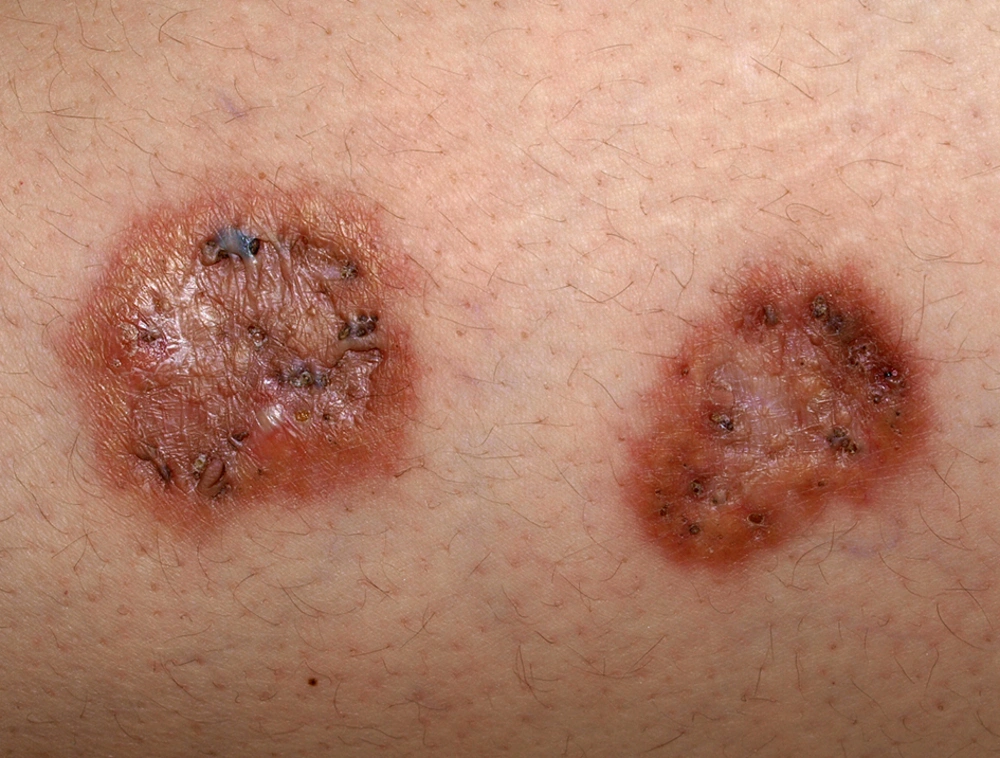
Granuloma annulare is a rare, chronic, self-limiting annular dermatosis with multifactorial etiology. In addition to diabetes, etiologic factors include atopy, thyroiditis, connective tissue disease and neoplasia. It affects women twice as often as men (32). Clinically, granuloma annulare presents with papules that tend to conflate and form annular plaques with a raised edge and a flat center. It occurs more frequently on the limbs and at the sites of trauma, with lesions being erythematous or with skin color. The course is usually asymptomatic but itching or burning is reported in some cases. The generalized variant of granuloma annulare (Figure 4) is associated with diabetes in 15% (32) to 33% of cases (33). Except for cases of spontaneous regressions, granuloma annulare is resistant to therapy. Retinoids and phototherapy have clinical effects in some patients.
3.2.9. Lichen Planus
Lichen planus is an inflammatory psychosomatic disorder, affecting mucous membranes and the skin. In a study, diabetes or impaired glucose tolerance was detected in about 25% of patients (34). The etiology is multifactorial, mainly associated with psychiatric, infectious, autoimmune or drug factors. The clinical picture includes erythematous or livid polygonal papules or plaques overlying the extensor surfaces of the limbs and the trunk. Wickham striae are usually observed on the closer inspection of the lesions. Leukokeratotic plaques or erosions on the oral and genital mucosa are also common findings. The triad of hypertension, oral lichen, and diabetes is known as Grinspan’s syndrome (35).
3.2.10. Vitiligo
Vitiligo is a chronic disease that occurs suddenly with the appearance of hypopigmented or a pigmented macule around the eyes, lips, elbows, knees, genitalia or trunk as a result of autoimmune melanocyte destruction in the skin. It affects about 1% of the population, more often women (36) and is most commonly associated with insulin-independent diabetes. The association of vitiligo, diabetes, thyroiditis, Addison's disease, myasthenia gravis, idiopathic thrombopenic purpura is known as multiple autoimmune syndrome (37).
Some authors stated that several dermatoses like hidradenitis suppurativa (38), glucagonoma (39), eruptive xanthoma (40), lipoid proteinosis (41), Kyrle disease (42), carotenoderma, and psoriasis vulgaris are more common in diabetes. The latter remains a controversy but these diseases represent metabolic disturbances. An interesting clinical observation in patients with diabetes is a discordance between the color of the hair, which turns gray with age, and that of the eyebrows, which remains darker in color (43).
3.3. Diabetes-Associated Skin Infections
Patients with diabetes are significantly more susceptible to bacterial and mycotic infections than the rest of the population. Infectious dermatoses are observed in about 20% of patients with diabetes (44), with mycotic infections being prevalent in frequency in comparison with bacterial and viral ones (45). Diabetes-associated infections are characterized by chronic recurrent course therapeutic resistance and a higher incidence of potentially life-threatening complications. Some authors propose that systemic mycotic infections caused by Zygomycetes spp. are typical for patients with uncontrolled diabetes (46). Viral, bacterial and mycotic infections most frequently associated with diabetes are summarized in Box 2.
| Common Infections |
|---|
| Viral infections |
| Herpes simplex |
| Herpes zoster |
| Bacterial Infections |
| Erysipelas |
| Cellulitis |
| Necrotic fasciitis |
| Fournier gangrene |
| Staphylococcal folliculitis |
| Furunculosis |
| Carbunculosis |
| Erythrasma |
| External otitis |
| Mycotic Infections |
| Yeast infections |
| Candida stomatitis |
| Candida balanoposthitis |
| Vulvovaginitis |
| Intertrigo |
| Erosio interdigitorum |
| Onychomycosis |
| Dermatophytic infections |
| Tinea |
| Onychomycosis |
| Systemic mycotic infections |
| Mucormycosis |
3.4. Skin Manifestations Triggered by Anti-Diabetic Therapy
The current treatment of diabetes is carried out with two groups of medicines: insulin and oral antidiabetic agents. Insulin is administered subcutaneously. It may be related, although very rarely, to acute hypersensitivity side effects, including urticaria, angioneurotic edema and anaphylactic shock and may sometimes be life-threatening. Lipodystrophy (lipohypertrophy or lipoatrophy) might occur at the injection site. The latter not only is an aesthetic problem but also severely disrupts insulin pharmacokinetics. Therefore, the injection site should be changed if this side effect occurs (47).
Oral antidiabetic agents are a growing heterogeneous group of drugs. Allergic skin reactions and photosensitivity are relatively more common in the group of sulfonylureas, chlorpropamide and tolbutamide. In combination with alcohol, chlorpropamide causes disulfiram-like reactions (facial flushing, headaches and nausea). In recent years, an increasing number of reports have been published on bullous pemphigoid (48-50) or mucous membrane pemphigoid (51), induced by dipeptidyl peptidase 4 inhibitors (vildagliptin, linagliptin and sitagliptin). Generalized skin eruption, urticaria and eczema were reported after the initiation of sodium-glucose cotransporters (ipragliflozin, dapagliflozin, canagliflozin, and empagliflozin) for diabetes (52).
4. Conclusions
Diabetes mellitus is associated with a wide range of dermatological disorders. The recognition of the cutaneous sins of diabetes is important for its early diagnosis and can help with adequate disease control. On the other hand, an active search for initial changes, such as xerosis, hyperkeratosis or skin infections and their appropriate management could help reduce the late, often severe complications of diabetes.
Although most dermatological conditions require specific treatment, a key rule is to improve glycemic control. Patient education and lifestyle change are essential for improving the quality of life of diabetic patients.