1. Context
Sporotrichosis is a common subcutaneous mycosis in Latin America, acquired through traumatic inoculation of the fungus via contaminated organic material. The host’s immune system plays a crucial role in responding against the disease and reducing the progression of the infection. Immune cells, including neutrophils, macrophages, and numerous innate and acquired defense mechanisms interact with each other for recognizing and orchestrating coordinated immune responses against the pathogen (1).
The innate immune system consists of several specific cells representing the first line of defense against many fungal infections including sporotrichosis. These innate immune mechanisms include a variety of cells and pattern recognition receptors that are important to control systemic infections (2). Different factors such as the site of the infection, the causal agent, and the immune system can modulate the antifungal reaction of the host. Infectious agents such as Sporothrix sp. benefit from the resources provided to them by the host as long as the damage suffered from the immune system is not strong enough to affect the viability of the microbe (3). Here, we investigated the main immune mechanisms against sporotrichosis.
2. Evidence Acquisition
2.1. The Importance of the Innate Immune System in Sporotrichosis
The innate immune system allows distinguishing the host’s cells (self) from foreign structures (non-self). The system is the first line of defense against external, mostly pathogenic, agents (3). A properly functioning immune system is necessary for decreasing predisposition to numerous infections (4). The main mechanisms of the innate immunity against sporotrichosis, determining susceptibility to the infection, will be reviewed.
Several studies have been conducted in congenitally athymic mice (nu/nu) to assess the role of T-cell mediated immune responses in sporotrichosis, showing greater susceptibility and death rates in the infections caused by S. schenckii (5). In animal models, S. schenckii infection was shown to be promptly limited by effectively and persistently controlling the fungal load.
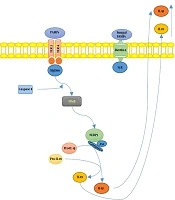
S. schenckiisensu stricto induced the release of several proinflammatory cytokines in mice experimental models (6), including TNFα and IL-6, at the infection site 24h after inoculation. However, in the case of S. brasiliensis, cytokines’ levels remained similar to uninfected mice. In Figure 1, we illustrated the hypothetical mechanisms of the interplay between Sporothrix sp. and the host’s immune system.
Summary of Sporotrix sp. and the host’s immune system interplay. Toll-like receptors (TLR) 2 and 4 recognize S. schenckii-derived mannans. The membrane-bound C-type lectins dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), macrophage-inducible C-type lectin, and macrophage mannose receptor (MR) recognize mannose-rich fungal structures. Moreover, the NOD-like receptor; NLRP3 (nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3), forms an inflammasome complex in combination with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and caspase 1, leading to interleukin-1β (IL-1β) and IL-18 production, contributing to the host’s immune defense against sporotrichosis.
The increased virulence of S. brasiliensis, as well as the decreased activity of the innate immune system and reduced Th1 response may promote the advanced stage of the infection. T cells favor Th17-mediated immune activity to control fungal infections while reducing excessive and damaging inflammatory responses through inducing T regulatory (Treg) cells (7). These results showed that S. brasiliensis had greater virulence compared with S. schenckii and S. globosa; on the other hand, S. mexicana and S. pallida (formerly S. albicans) showed low or no virulence (7).
2.2. Importance of Reactive Oxygen and Nitrogen Species
Macrophages are specialized cells against different infectious agents, including fungi. Macrophages’ functions involve phagocytosis, antigen processing and presentation, and ultimately linking the innate immunity with adaptive immunity (8). In addition to phagocytosis, macrophages produce substances such as enzymes, complement components, and coagulation factors (9), as well as potent inflammatory mediators like reactive oxygen species (ROS) and reactive nitrogen species (RNS) (10). The relevance of these ROS (mainly hydrogen peroxide, H2O2) in the microbicidal activity of macrophages has been suggested in previous studies (11); moreover, macrophage activity is suppressed by free radicals, as well as superoxide dismutase (related to ROS) and catalase and NO (related to RNS) (12).
An imbalance has been observed between immune responses to infections and the virulence of the agent. This imbalance is mediated through the NO released by macrophages; one consequence of which includes lower susceptibility to the infection (13).
2.3. Importance of Pattern Recognition Receptors
Importance of pattern recognition receptors (PRRs) are classes of innate immunity receptors, that are often produced during cellular damage or early phases of infections. These include Toll-like (TLRs) and NOD type receptors (RTNs). The primary function of PRRs is the recognition of various pathogens, modulating stress signaling (14).
2.4. Importance of TLRs and Phagocytosis
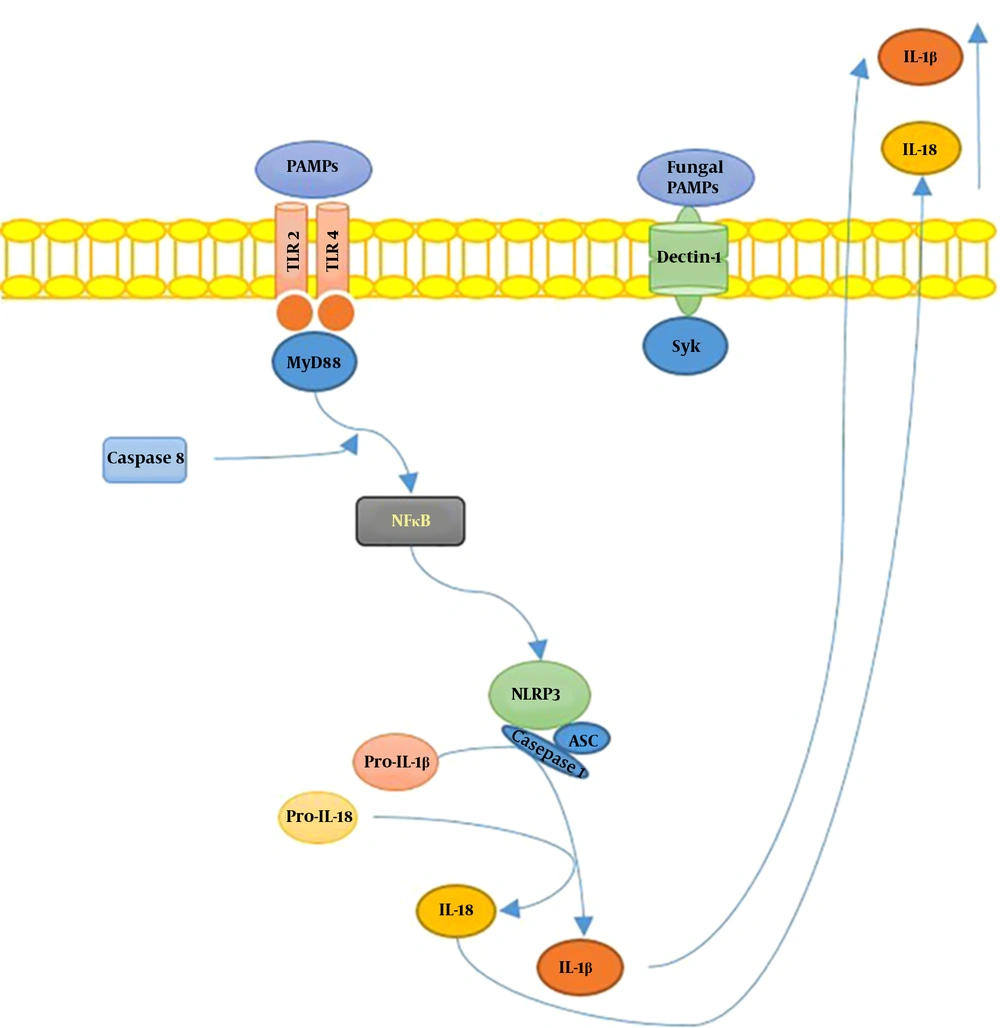
TLRs were first identified in Drosophila melanogaster and suggested to control the activity and progression of several mycoses (14). The engagement of TLRs with their agonists favors the dimerization of their extra and intracellular domains, leading to signal transduction through the myeloid differentiation primary response protein 88 (MyD88) and the activation of adaptive immune responses (15). Immune recognition of antigens on the surface of most fungi is mainly carried out by TLR4 and, albeit in a smaller proportion, TLR2/1, and TLR2/6 heterodimers. During fungal infections, the functions of TLRs can be antagonistic but complementary. TLR4 regulates the Th1 response and influences the development of Th17, while TLR2 promotes the differentiation of Treg cells by regulating Th1 and Th17 activities (16, 17).
Phagocytosis constitutes the first line of immune defense, allowing for the sampling of antigens and the processing of invading pathogens (8). Phagocytosis is directed by several fully identified receptors including FC receptors, integrins, and complement receptors. Moreover, other receptors may be involved in the binding of antigens, potentiating their internalization (8). Among PRRs, mannose receptor (MRs, like Dectin-1, are phagocytic receptors with no inflammatory signaling; however, they co-modulate these signals and enhance phagocytosis in combination with other PRRs (such as TLRs 3, 7, 8 and 9) (18). Recent studies have shown the role of TLR2 in potentiating S. schenckii phagocytosis and inducing the production of inflammatory mediators by macrophages (19, 20).
2.5. Importance of RTNs and the Inflammasome
RTNs are cytosolic PRRs forming pentameric/heptameric protein structures known as inflammasomes. Infectious agents that surpass the host’s surface receptors are recognized by RTNs (21, 22). Inflammasome acts as a regulator of IL-18-mediated inflammation, contributing to the defense against infectious diseases; these mechanisms may enhance the antimicrobial characteristics of inflammatory cells, mainly phagocytes, activating Th1/Th17 responses (23).
In a recent report by Goncalves et al. (24), the role of inflammasomes in S. schenckii infection was determined, indicating that the inflammasomes assembled with NLRP3, an intracellular sensor that detects a broad range of microbial motifs (25), play a protective role against the infection. This function is related to the activation of pro-inflammatory mediators and IL-1/IL-17 induced T helper responses, linking the recognition of the fungus via innate immunity receptors to subsequent adaptive immune responses (25).
2.6. Importance of Macrophage Activation Profile
Macrophages evolve into two types of phenotypes (26) essential to adequate immune responses against several pathogens. These include activated “classic” (M1) and “alternative” (M2) cell types. The first may cause tissue damage and directly influence sporotrichosis through “producing high levels of IL-12and regulating the activity of nitric oxide (NO) synthase (27). On the other hand, M2 macrophages produce high amounts of IL10, regulate the activity of the arginase I enzyme, and promote tissue repair by inducing angiogenesis and the remodeling of the affected tissue (28). In an experimental model of sporotrichosis, both M1 and M2 macrophages were identified during the active phase of the disease. After stimulation with a cell wall-derived peptide-polysaccharide, the production of IL-10 and TFG-β increased during the infection (29).
2.7. Importance of Dendritic Cell (DC) Activation Profile
Activation of DCs regulates the interaction between pro- and anti-inflammatory mechanisms necessary for immune regulation (30). The specific function of these cells in sporotrichosis has not been entirely elucidated. Uenotsuchi et al. (30) showed that DCs derived from human monocytes expressed a unique cellular function when they were exposed to yeasts, and the conidia of S. schenckii derived from patients with either visceral or cutaneous sporotrichosis.
In another study, bone marrow-derived dendritic cells (BMDCs) were stimulated with either complete fungi or the exoantigen of S. schenckii; the recognition of these antigens by DCs (plasticity) triggered T cell-mediated immune mechanisms (31).
2.8. Importance of Interactions Among Immune Responses in Sporotrichosis
The interplay between innate and adaptive immune responses is important for more robust and directed responses leading to the control of infectious agents. Many specific antifungal cellular and humoral immune pathways are directed against S. schenckii, playing vital roles in protecting the host against the fungus (32).
The severity of sporotrichosis depends mainly on the extent of immune responses; immunosuppressed subjects develop disseminated clinical forms whereas immunocompetent subjects generally develop localized manifestations (24). This is probably due to variable T-cell mediated immune responses, which are essential to define the clinical severity of sporotrichosis (33).
IL-12 induces the proliferation of T cells and natural killer (NK) cells and is an essential mediator of immune responses against not only viruses but also fungi as shown by the growing evidence on the effects of some fungi such as S. schenckii on the maturation of NK cells and the production of proinflammatory cytokines (34). The development of Th1 immunity is primarily mediated by IL-12 and IFNγ (27). On the other hand, TNFα is an essential cytokine with a critical function in several acute and chronic inflammatory diseases (35). This can be explained by its ability to induce proinflammatory cytokines, mainly IL-1 (36).
IFNγ is a potent trigger for activating macrophages, and its local production in pathological conditions prolongs the course of diseases (37). Also, the macrophages activated by IFNγ produce different cytokines that control the progression of infectious agents; among these are TNFα, as well as IL6 and 12. IFNγ also induces NO production by stimulating macrophages and modulates T-cell proliferation and function mainly through regulating IL-12 production (38). Also, IFNγ improves the antigen presentation activity of macrophages and promotes different stimuli to increase the activity of Th1 lymphocytes, thereby enhancing not only innate but also adaptive immunity. IL4 and IL10 augment the humoral immunity via stimulating specialized cells such as eosinophils and mast cells, induce the differentiation of B cells to plasma cells, and finally trigger the production of IgE. In the initial phase of sporotrichosis, increased levels of IL4 may delay the onset of the Th1 response by inhibiting the production of IFNγ while in the later stages of the disease, the infection can be drastically limited by the intervention of cytokines such as IL4 (39). This initial increase in IL4 levels is maintained during the advanced stages of the disease, which in combination with elevated IgG titers, it reflects the involvement of Th2 immunity in advanced stages of sporotrichosis, at least in animal models (40).
2.9. Importance of Apoptosis and Th17 Response in Sporotrichosis
Apoptosis is a mechanism for cellular death, in which cells are removed without an evident sign of inflammation. Apoptosis may follow an infectious process either as an escape mechanism for the infectious agent or a strategy to prevent the spread of the infection (41). Fernandes et al. (42) showed that the immunosuppression associated with sporotrichosis and susceptibility to a systemic disease in a murine model were probably related to an increase in NO production, leading to the apoptosis of splenic cells, the suppression of the immune response, and the high production of IL-10 and TNFα, as observed in several infectious diseases including sporotrichosis (43). The above-mentioned findings seem to explain the depletion of cellular immunity observed in the acute phase of sporotrichosis (44).
2.10. Importance of the Th17 Response in Sporotrichosis
Infection-induced apoptosis is involved in the induction and development of Th17 responses (45); however, the role of this pathway in sporotrichosis pathogenesis is not clear yet. The essential role of Th17 cells in the immunoprotection against agents such as bacteria and fungi, as well as the production of IL17, which is necessary for homeostasis of the mucosa, has been shown in previous studies. IL17 also increases the recruitment of neutrophils and Th1 cells and induces the production of proinflammatory cytokines by epithelial cells (46).
A previous study evaluated the immunogenic potential of the fungus (S. schenckii) and its exoantigen. The exoantigen was sufficient to induce the release of proinflammatory cytokines (31). The exposure of BMDCs to the entire fungus induced the release of significant levels of IL-12 and IL-6; however, the release of TGFβ was minimal. IL-23, which is mainly induced by the exoantigen of S. schenckii, in addition to inducing high levels of IL-6 and decreasing IL-12, which inhibits the differentiation of Th17 cells, results in an intense release of IL-17 in the culture medium of BMDCs and splenic lymphocytes (31).
3. Conclusions
The recognition of S. schenckii via TLR4 leads to potent inflammatory reactions. TLR2 regulates inflammation and may represent an escape mechanism of the fungus. Simultaneously, the activation of inflammasomes and apoptosis by sporotrichosis regulates immune responses, mainly Th17-induced reactions, against S. schenckii. Nevertheless, these aspects are still under study. Currently, the important points related to the innate and adaptive immune responses against the infectious agent are under evaluation both in vivo and in vitro to characterize the behavior of the fungus in different experimental models.